Abstract
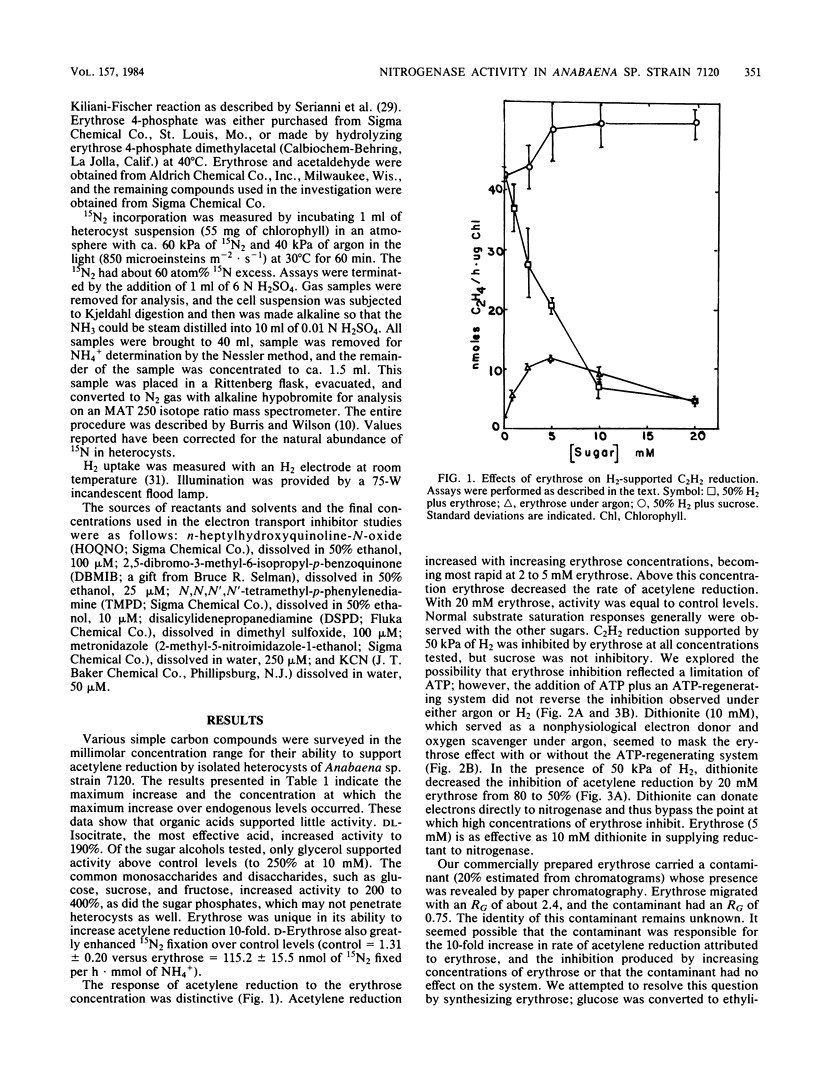
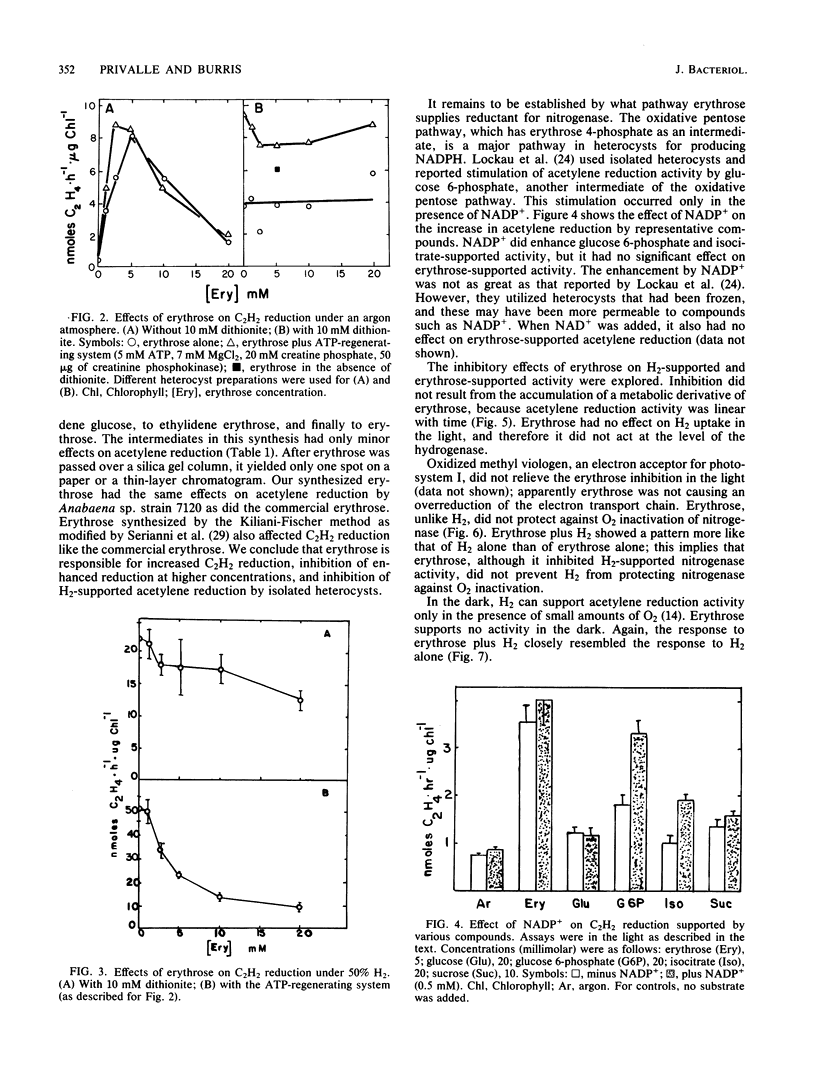
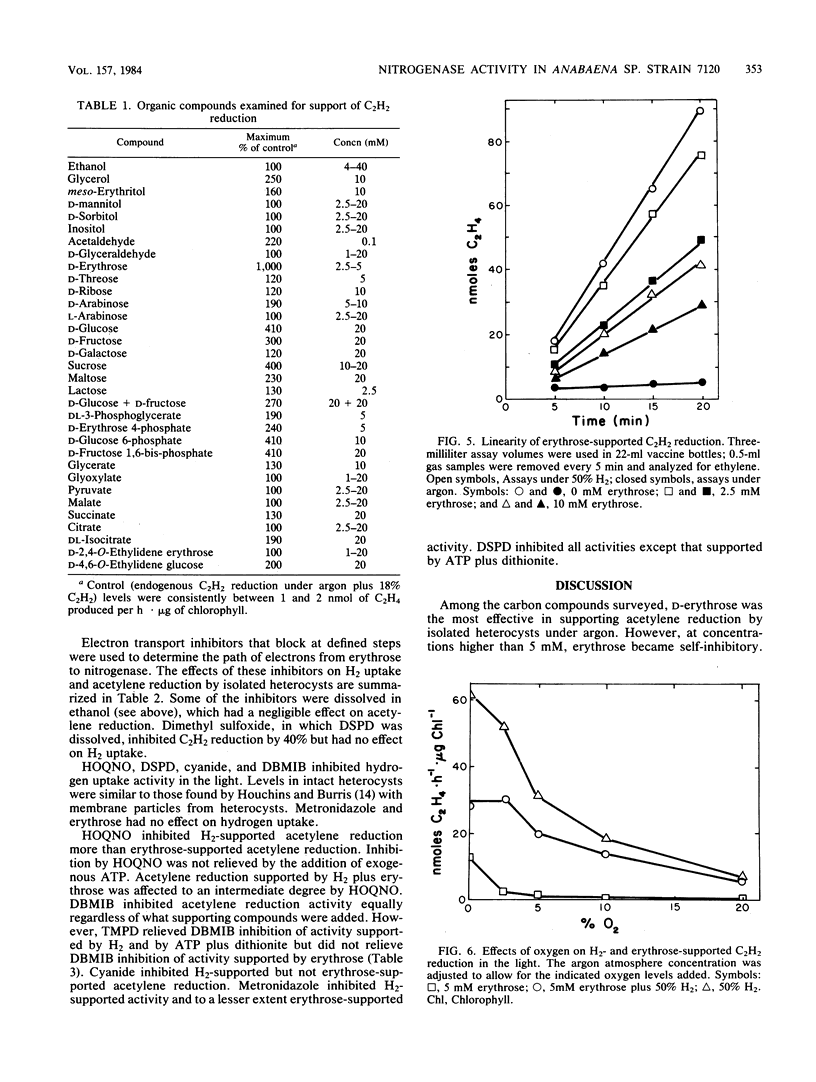
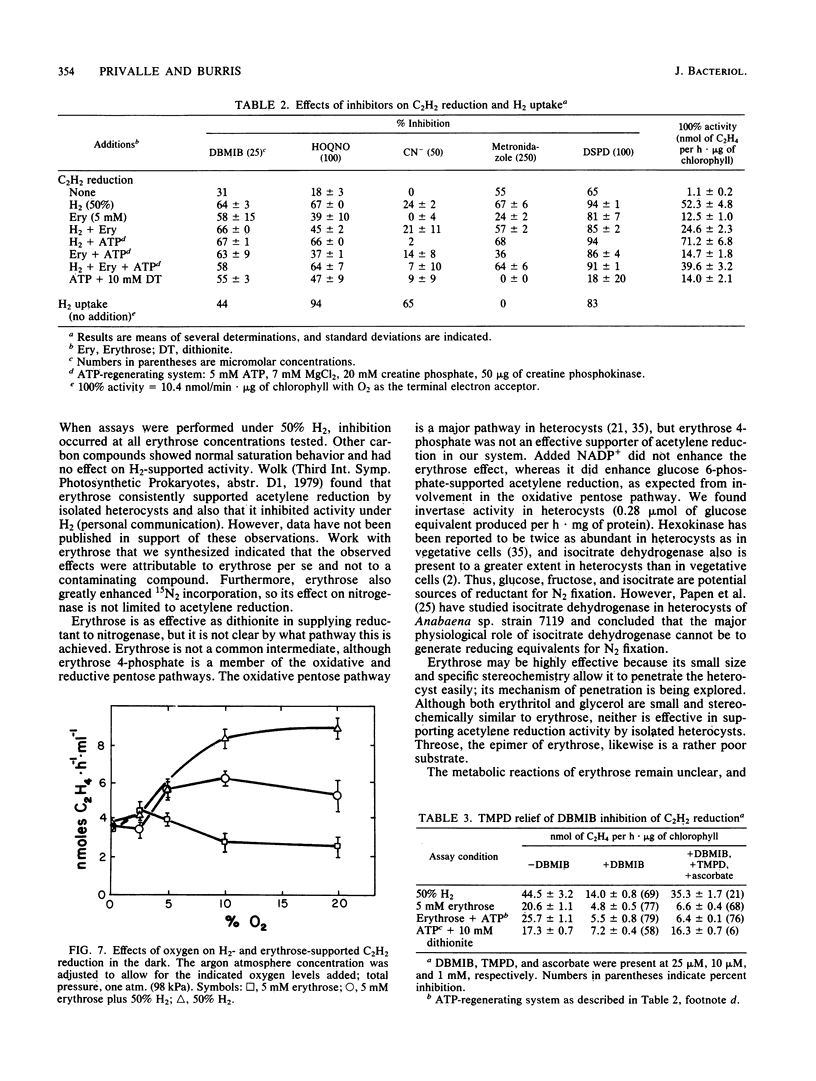
Among organic compounds tested for their ability to support nitrogenase activity in isolated heterocysts of Anabaena sp. strain 7120 under argon, D-erythrose (5 mM) was unique in supporting acetylene reduction at 10 times the control rates. Higher concentrations of D-erythrose exhibited substrate inhibition. At 50 kPa of H2, all concentrations of D-erythrose inhibited H2-supported acetylene reduction. The effects of D-erythrose on nitrogenase activity were explored. Erythrose enhanced 15N2 incorporation by heterocysts, but NADP+ did not enhance erythrose-supported acetylene reduction. H2 protected nitrogenase from O2 inactivation, but erythrose did not; erythrose did not counter protection by H2. Tests with inhibitors of electron transport showed that erythrose-supported acetylene reduction requires electron flow through ferredoxin, a b-type cytochrome, and a 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone-sensitive transfer agent whose electron flow is not mediated through the plastoquinone and Rieske iron protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. B., Arnon D. I. Studies on Nitrogen-Fixing Blue-Green Algae. I. Growth and Nitrogen Fixation by Anabaena Cylindrica Lemm. Plant Physiol. 1955 Jul;30(4):366–372. doi: 10.1104/pp.30.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer J. R., Dutton P. L., Prince R. C., Crofts A. R. The role of the Rieske iron-sulfur center as the electron donor to ferricytochrome c2 in Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1980 Oct 3;592(3):445–460. doi: 10.1016/0005-2728(80)90091-2. [DOI] [PubMed] [Google Scholar]
- Convent B., Briquet M. Properties of 3-(3, 4-dichlorophenyl)-1, 1-dimethylurea and other inhibitors of the cytochrome bc1 segment of the mitochondrial respiratory chain in Saccharomyces cerevisiae. Eur J Biochem. 1978 Jan 16;82(2):473–481. doi: 10.1111/j.1432-1033.1978.tb12041.x. [DOI] [PubMed] [Google Scholar]
- Haury J. F., Spiller H. Fructose uptake and influence on growth of and nitrogen fixation by Anabaena variabilis. J Bacteriol. 1981 Jul;147(1):227–235. doi: 10.1128/jb.147.1.227-235.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchins J. P., Burris R. H. Light and dark reactions of the uptake hydrogenase in anabaena 7120. Plant Physiol. 1981 Sep;68(3):712–716. doi: 10.1104/pp.68.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley B. C., Nicholas D. J. Inhibition of nitrogenase activity by metronidazole in rhodopseudomonas capsulata. Arch Microbiol. 1981 Jul;129(5):344–348. doi: 10.1007/BF00406459. [DOI] [PubMed] [Google Scholar]
- Laasch N., Kaiser W., Urbach W. Effects of disalicylidenepropanediamines on photosynthetic electron transport of isolated spinach chloroplasts. Plant Physiol. 1979 Apr;63(4):605–608. doi: 10.1104/pp.63.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex M., Carr N. G. The metabolism of glucose by heterocysts and vegetative cells of Anabaena cylindrica. Arch Microbiol. 1974;101(2):161–167. doi: 10.1007/BF00455936. [DOI] [PubMed] [Google Scholar]
- Ljones T. Nitrogen fixation and bioenergetics: the role of ATP in nitrogenase catalysis. FEBS Lett. 1979 Feb 1;98(1):1–8. doi: 10.1016/0014-5793(79)80138-6. [DOI] [PubMed] [Google Scholar]
- Lockau W., Peterson R. B., Wolk C. P., Burris R. H. Modes of reduction of nitrogen in heterocysts isolated from Anabaena species. Biochim Biophys Acta. 1978 May 10;502(2):298–308. doi: 10.1016/0005-2728(78)90051-8. [DOI] [PubMed] [Google Scholar]
- Papen H., Neuer G., Refaian M., Bothe H. The isocitrate dehydrogenase from cyanobacteria. Arch Microbiol. 1983 Jan;134(1):73–79. doi: 10.1007/BF00429411. [DOI] [PubMed] [Google Scholar]
- Peschek G. A., Schmetterer G. Evidence for plastoquinol-cytochrome f/b-563 reductase as a common electron donor to P700 and cytochrome oxidase in cyanobacteria. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1188–1195. doi: 10.1016/0006-291x(82)92126-x. [DOI] [PubMed] [Google Scholar]
- Rivera-Ortiz J. M., Burris R. H. Interactions among substrates and inhibitors of nitrogenase. J Bacteriol. 1975 Aug;123(2):537–545. doi: 10.1128/jb.123.2.537-545.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. V., Noy R. J., Evans M. C. Physiological electron donor systems to the nitrogenase of the blue-green alga Anabaena cylindrica. Biochim Biophys Acta. 1971 Nov 2;253(1):104–109. doi: 10.1016/0005-2728(71)90238-6. [DOI] [PubMed] [Google Scholar]
- Sweet W. J., Houchins J. P., Rosen P. R., Arp D. J. Polarographic measurement of H2 in aqueous solutions. Anal Biochem. 1980 Sep 15;107(2):337–340. doi: 10.1016/0003-2697(80)90393-0. [DOI] [PubMed] [Google Scholar]
- Tetley R. M., Bishop N. I. The differential action of metronidazole on nitrogen fixation, hydrogen metabolism, photosynthesis and respiration in Anabaena and Scenedesmus. Biochim Biophys Acta. 1979 Apr 11;546(1):43–53. doi: 10.1016/0005-2728(79)90168-3. [DOI] [PubMed] [Google Scholar]
- Winkenbach F., Wolk C. P. Activities of enzymes of the oxidative and the reductive pentose phosphate pathways in heterocysts of a blue-green alga. Plant Physiol. 1973 Nov;52(5):480–483. doi: 10.1104/pp.52.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk C. P. Movement of carbon from vegetative cells to heterocysts in Anabaena cylindrica. J Bacteriol. 1968 Dec;96(6):2138–2143. doi: 10.1128/jb.96.6.2138-2143.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



