Abstract
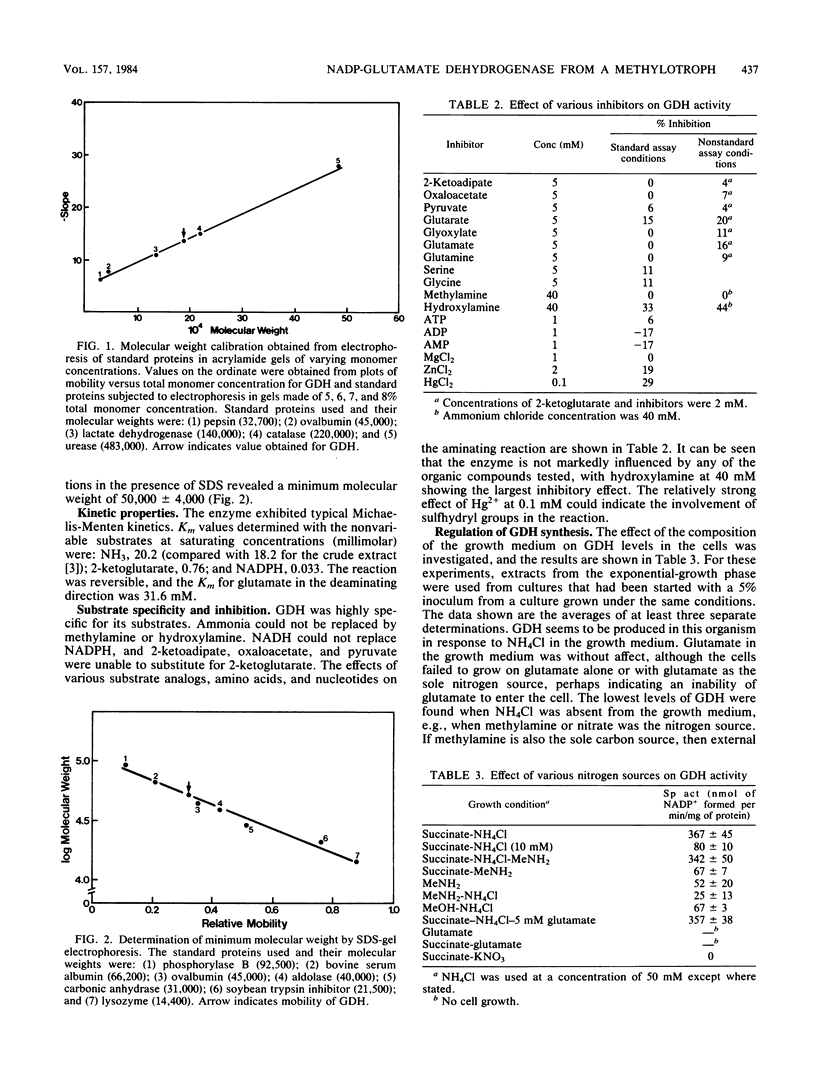
The NADP-dependent glutamate dehydrogenase (EC 1.4.1.4.) elaborated by the methylotrophic bacterium Pseudomonas sp. strain AM1 when growing on succinate and ammonium chloride was studied. The enzyme, which has a pH optimum of 9.0, was purified 140-fold and shown to have Km values of 20.2 mM, 0.76 mM, 0.033 mM, and 31.6 mM for ammonia, alpha-ketoglutarate, NADPH, and glutamate, respectively. The native molecular weight was determined by polyacrylamide gel electrophoresis to be 190,000, and electrophoresis under denaturing conditions in the presence of sodium dodecyl sulfate revealed a minimum molecular weight of 50,000. The enzyme was highly specific; NADH was unable to replace NADPH in the reaction, various alpha-keto acids could not replace alpha-ketoglutarate, and neither methylamine nor hydroxylamine could substitute for ammonia. Glutamate dehydrogenase was synthesized by the bacteria only when ammonia was its nitrogen source and was repressed if methylamine or nitrate were provided as sources of nitrogen instead of ammonia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Gharawi A., Moore D. Factors affecting the amount and the activity of the glutamate dehydrogenases of Coprinus cinereus. Biochim Biophys Acta. 1977 Jan 24;496(1):95–102. doi: 10.1016/0304-4165(77)90118-0. [DOI] [PubMed] [Google Scholar]
- Bellion E., Kent M. E., Aud J. C., Alikhan M. Y., Bolbot J. A. Uptake of methylamine and methanol by Pseudomonas sp. strain AM1. J Bacteriol. 1983 Jun;154(3):1168–1173. doi: 10.1128/jb.154.3.1168-1173.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellion E., Khan M. Y., Romano M. J. Transport of methylamine by Pseudomonas sp. MA. J Bacteriol. 1980 Jun;142(3):786–790. doi: 10.1128/jb.142.3.786-790.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellion E., Wayland L. Methylamine uptake in Pseudomonas species strain MA: utilization of methylamine as the sole nitrogen source. J Bacteriol. 1982 Jan;149(1):395–398. doi: 10.1128/jb.149.1.395-398.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Coulton J. W., Kapoor M. Studies on the kinetics and regulation of glutamate dehydrogenase of Salmonella typhimurium. Can J Microbiol. 1973 Apr;19(4):439–450. doi: 10.1139/m73-072. [DOI] [PubMed] [Google Scholar]
- Epstein I., Grossowicz N. Purification and properties of glutamate dehydrogenase from a thermophilic bacillus. J Bacteriol. 1975 Jun;122(3):1257–1264. doi: 10.1128/jb.122.3.1257-1264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hemmilä I. A., Mäntsälä P. I. Purification and properties of glutamate synthase and glutamate dehydrogenase from Bacillus megaterium. Biochem J. 1978 Jul 1;173(1):45–52. doi: 10.1042/bj1730045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper A. B., Hansen J., Bell R. Characterization of glutamate dehydrogenase from the ammonia-oxidizing chemoautotroph Nitrosomonas europaea. J Biol Chem. 1967 Jan 25;242(2):288–296. [PubMed] [Google Scholar]
- Janssen D. B., op den Camp H. J., Leenen P. J., van der Drift C. The enzymes of the ammonia assimilation in Pseudomonas aeruginosa. Arch Microbiol. 1980 Feb;124(2-3):197–203. doi: 10.1007/BF00427727. [DOI] [PubMed] [Google Scholar]
- Krämer J. NAD and NADP-dependent glutamate dehydrogenase in Hydrogenomonas H 16. Arch Mikrobiol. 1970;71(3):226–234. doi: 10.1007/BF00410156. [DOI] [PubMed] [Google Scholar]
- Loginova N. V., Govorukhina N. I., Trotsenko Iu A. Fermenty assimiliatsii ammoniia u bakterii s razlichnymi putiami C1-metabolizma. Mikrobiologiia. 1982 Jan-Feb;51(1):38–42. [PubMed] [Google Scholar]
- LéJohn H. B., McCrea B. E. Evidence for two species of glutamate dehydrogenases in Thiobacillus novellus. J Bacteriol. 1968 Jan;95(1):87–94. doi: 10.1128/jb.95.1.87-94.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. I. Isolation and characterization of Pseudomonas AM 1. Biochem J. 1961 Dec;81:465–469. doi: 10.1042/bj0810465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phibbs P. V., Jr, Bernlohr R. W. Purification, properties, and regulation of glutamic dehydrogenase of Bacillus licheniformis. J Bacteriol. 1971 May;106(2):375–385. doi: 10.1128/jb.106.2.375-385.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto N., Kotre A. M., Savageau M. A. Glutamate dehydrogenase from Escherichia coli: purification and properties. J Bacteriol. 1975 Nov;124(2):775–783. doi: 10.1128/jb.124.2.775-783.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiio I., Ozaki H. Regulation of nicotinamide adenine dinucleotide phosphate-specific glutamate dehydrogenase from Brevibacterium flavum, a glutamate-producing bacterium. J Biochem. 1970 Nov;68(5):633–647. doi: 10.1093/oxfordjournals.jbchem.a129397. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Windass J. D., Worsey M. J., Pioli E. M., Pioli D., Barth P. T., Atherton K. T., Dart E. C., Byrom D., Powell K., Senior P. J. Improved conversion of methanol to single-cell protein by Methylophilus methylotrophus. Nature. 1980 Oct 2;287(5781):396–401. doi: 10.1038/287396a0. [DOI] [PubMed] [Google Scholar]
- Yarrison G., Young D. W., Choules G. L. Glutamate dehydrogenase from Mycoplasma laidlawii. J Bacteriol. 1972 May;110(2):494–503. doi: 10.1128/jb.110.2.494-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



