Abstract
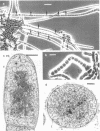
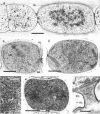
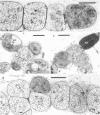
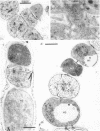
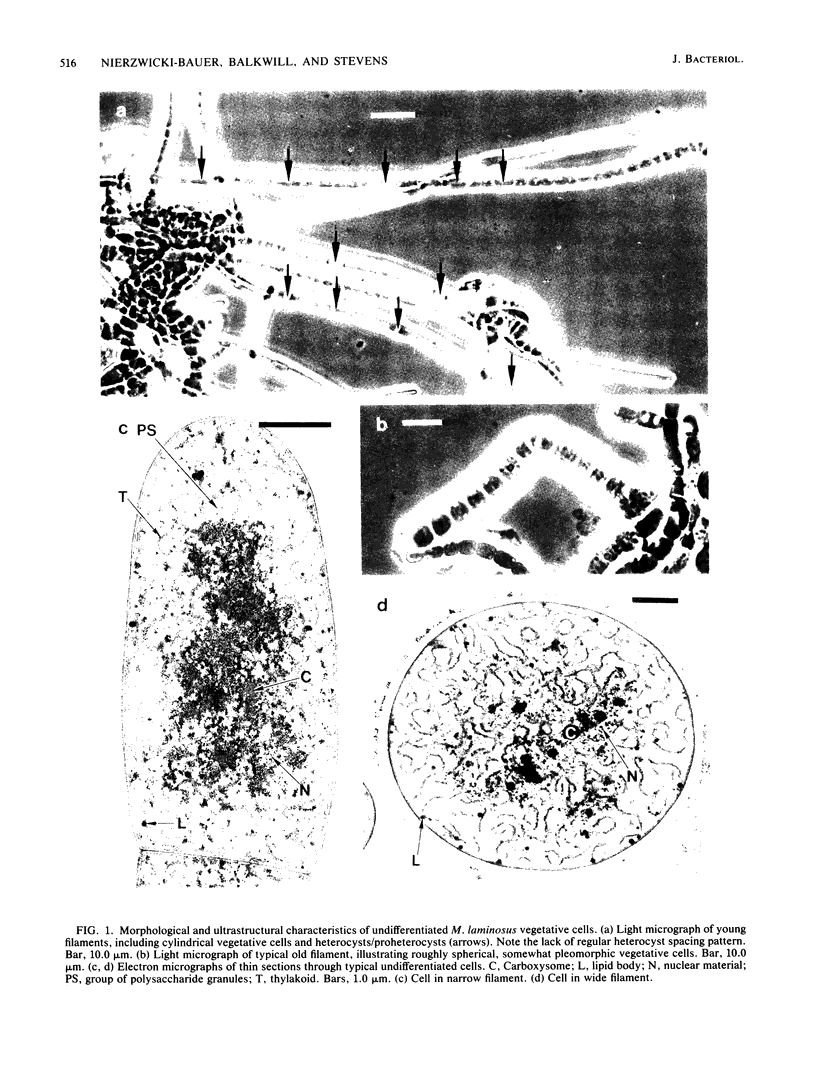
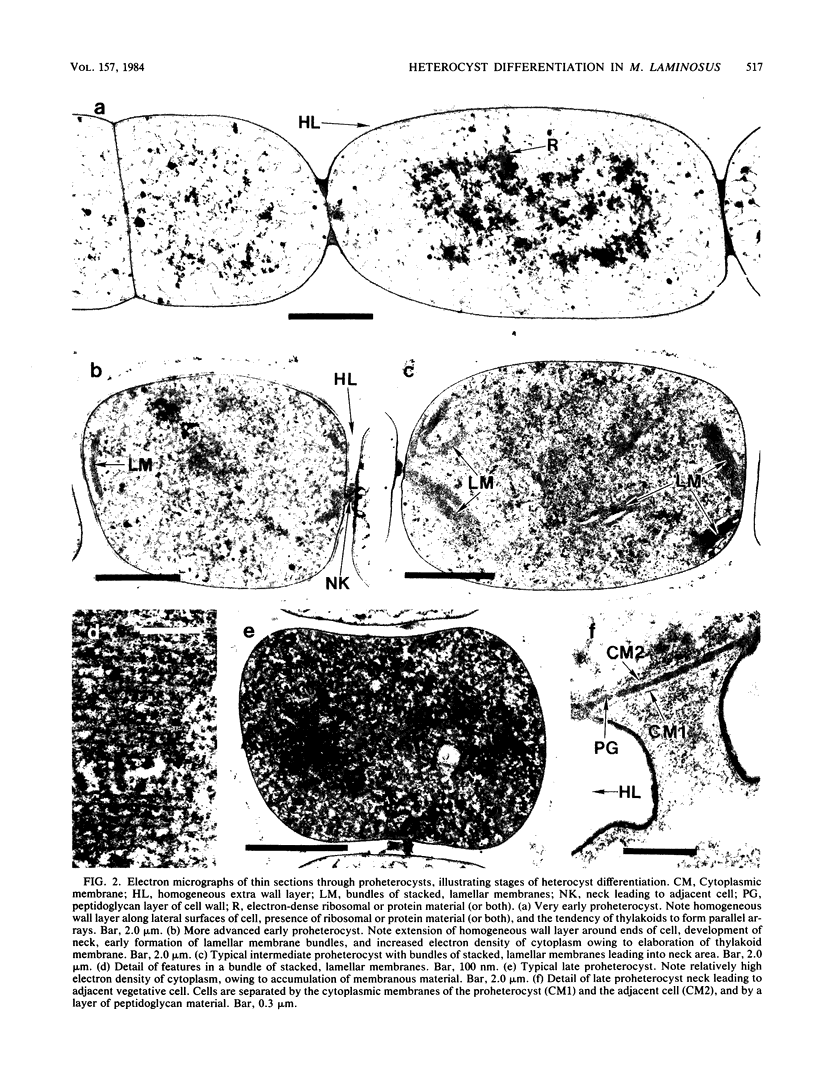
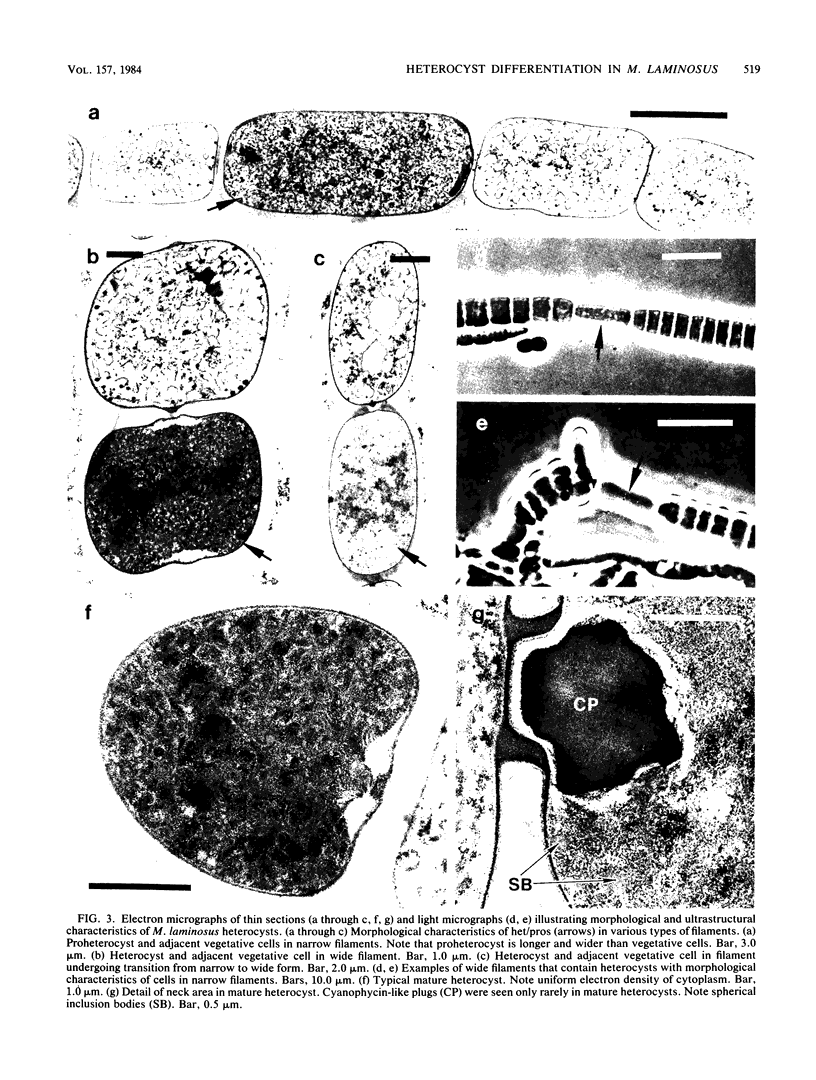
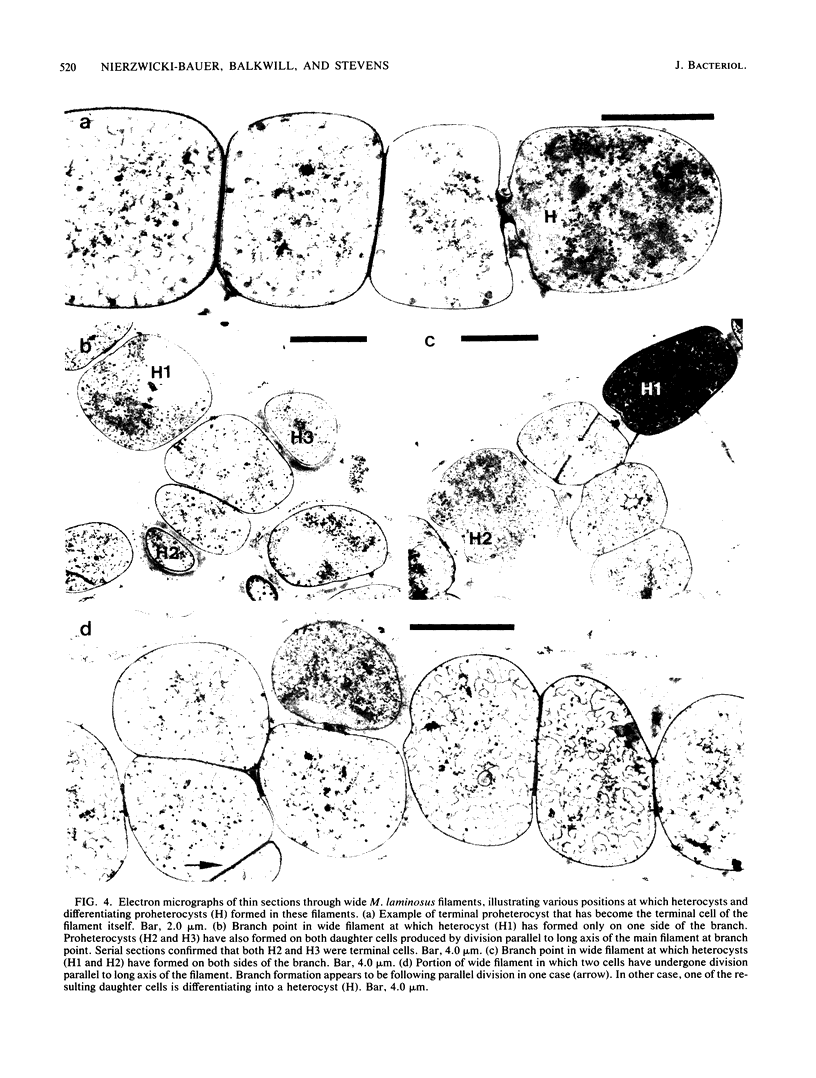
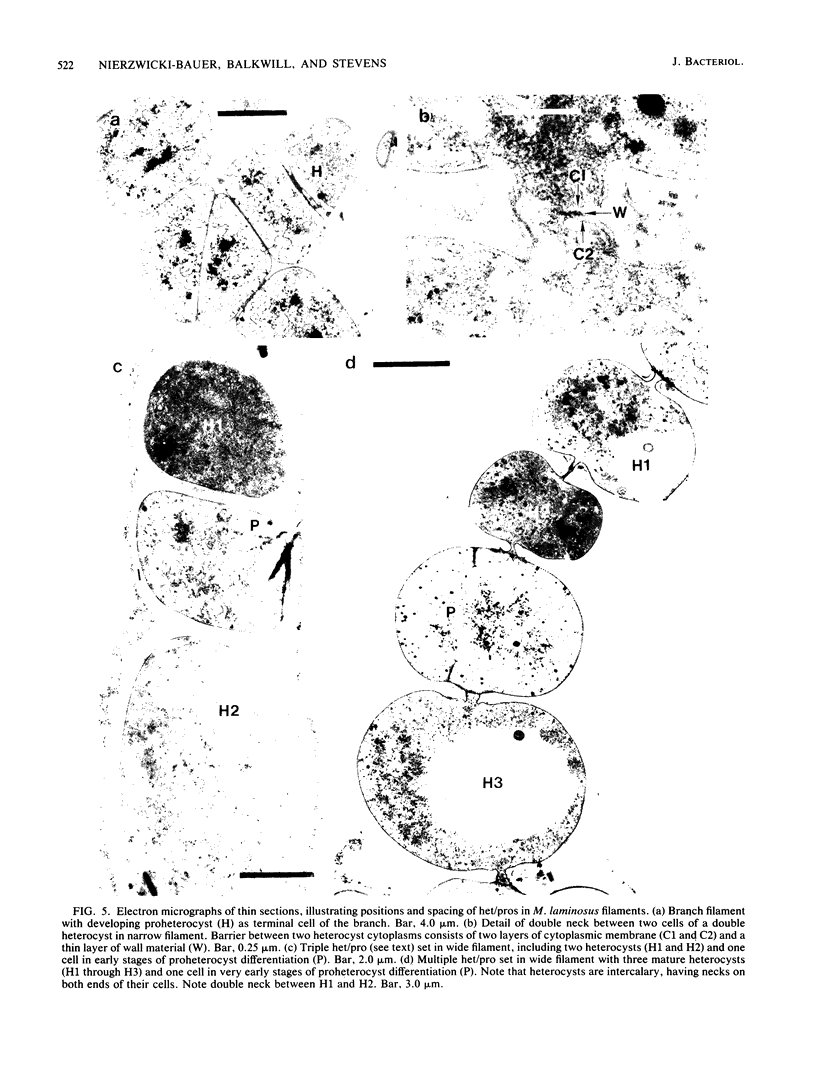
The morphological and ultrastructural aspects of heterocyst differentiation in the branching, filamentous cyanobacterium Mastigocladus laminosus were examined with light and electron microscopy. The earliest differentiation stages involved cytoplasmic changes, including (i) rapid degradation of carboxysomes, (ii) degradation of polysaccharide granules, and (iii) accumulation of electron-dense ribosomal or protein material (or both). Intermediate differentiation stages involved synthesis of a homogeneous extra wall layer, development of necks leading to adjacent cells, and elaboration of a complex system of intracytoplasmic membranes. Late differentiation stages included further development of necks and continued elaboration of membranes. Mature heterocysts possessed a uniformly electron-dense cytoplasm that contained large numbers of closely packed membranes, some of which were arranged in lamellar stacks. Mature heterocysts lacked all of the inclusion bodies present in undifferentiated vegetative cells, but contained a number of unusual spherical inclusions of variable electron density. Cells in both narrow and wide filaments were capable of differentiating. No regular heterocyst spacing pattern was observed in the narrow filaments; the number of vegetative cells between consecutive heterocysts of any given filament varied by a factor of 10. Heterocysts developed at a variety of locations in the wide, branching filaments, although the majority of them were situated adjacent to branch points. M. laminosus displayed a marked tendency to produce sets of adjacent heterocysts or proheterocysts (or both) that were not separated from each other by vegetative cells. Groups of four or more adjacent heterocysts or proheterocysts occurred frequently in wide filaments, and in some of these filaments virtually all of the cells appeared to be capable of differentiating into heterocysts.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. G., Carr N. G. The developmental biology of heterocyst and akinete formation in cyanobacteria. Crit Rev Microbiol. 1981;9(1):45–100. doi: 10.3109/10408418109104486. [DOI] [PubMed] [Google Scholar]
- Chao L., Bowen C. C. Purification and properties of glycogen isolated from a blue-green alga, Nostoc muscorum. J Bacteriol. 1971 Jan;105(1):331–338. doi: 10.1128/jb.105.1.331-338.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N. Phycobilisomes: structure and dynamics. Annu Rev Microbiol. 1982;36:173–198. doi: 10.1146/annurev.mi.36.100182.001133. [DOI] [PubMed] [Google Scholar]
- Isogai Y., Iida A., Mochizuki K., Okabe H., Yokose T. [Therapeutic and pathophysiologic consideration on diabetic microangiopathy from the view point of the blood rheology (author's transl)]. Horumon To Rinsho. 1980 Feb;28(2):149–154. [PubMed] [Google Scholar]
- Kulasooriya S. A., Lang N. J., Fay P. The heterocysts of blue-green algae. 3. Differentiation and nitrogenase activity. Proc R Soc Lond B Biol Sci. 1972 Jun 6;181(1063):199–209. doi: 10.1098/rspb.1972.0046. [DOI] [PubMed] [Google Scholar]
- Mitchison G. J., Wilcox M. Alteration in heterocyst pattern of Anabaena produced by 7-azatryptophan. Nat New Biol. 1973 Dec 19;246(155):229–233. doi: 10.1038/newbio246229a0. [DOI] [PubMed] [Google Scholar]
- Mitchison G. J., Wilcox M., Smith R. J. Measurement of an inhibitory zone. Science. 1976 Feb 27;191(4229):866–868. doi: 10.1126/science.814620. [DOI] [PubMed] [Google Scholar]
- RIS H., SINGH R. N. Electron microscope studies on blue-green algae. J Biophys Biochem Cytol. 1961 Jan;9:63–80. doi: 10.1083/jcb.9.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. M. Inclusion bodies of prokaryotes. Annu Rev Microbiol. 1974;28(0):167–187. doi: 10.1146/annurev.mi.28.100174.001123. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stevens S. E., Paone D. A. Accumulation of Cyanophycin Granules as a Result of Phosphate Limitation in Agmenellum quadruplicatum. Plant Physiol. 1981 Apr;67(4):716–719. doi: 10.1104/pp.67.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox M., Mitchison G. J., Smith R. J. Mutants of Anabaena cylindrica altered in heterocyst spacing. Arch Microbiol. 1975 May 5;103(3):219–223. doi: 10.1007/BF00436353. [DOI] [PubMed] [Google Scholar]
- Wilcox M., Mitchison G. J., Smith R. J. Pattern formation in the blue-green alga Anabaena. II. Controlled proheterocyst regression. J Cell Sci. 1973 Nov;13(3):637–649. doi: 10.1242/jcs.13.3.637. [DOI] [PubMed] [Google Scholar]
- Wilcox M., Mitchison G. J., Smith R. J. Pattern formation in the blue-green alga, Anabaena. I. Basic mechanisms. J Cell Sci. 1973 May;12(3):707–723. doi: 10.1242/jcs.12.3.707. [DOI] [PubMed] [Google Scholar]
- Wolk C. P. Physiology and cytological chemistry blue-green algae. Bacteriol Rev. 1973 Mar;37(1):32–101. doi: 10.1128/br.37.1.32-101.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]