Abstract
Retinoids are crucial regulators of a wide variety of processes in both developing and adult animals. These effects are thought to be mediated by the retinoic acid (RA) receptors and the retinoid X receptors (RXRs). We have identified an additional retinoid-activated receptor that is neither a retinoic acid receptors nor an RXR. RXR-interacting protein 14 (RIP14), a recently described orphan member of the nuclear receptor superfamily, can be activated by either all-trans-RA (tRA) or the synthetic retinoid TTNPB {[E]-4-[2-(5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-naphthalenyl)propen-1-yl]benzoic acid}. RIP14 binds to DNA as a heterodimer with RXR. In the presence of either tRA or TTNPB, the addition of 9-cis-RA or the RXR-specific agonist LG1069 {4-[1-(3, 5, 5, 8, 8-pentamethyl-5, 6, 7, 8-tertrahydro-2-naphthyl)ethenyl]benzoic acid} results in additional activation. Mutations of the ligand-dependent transcriptional activation functions indicate that TTNPB activates the RIP14 component of the RIP14–RXR heterodimer, that 9-cis-RA and LG1069 activate RXR, and that tRA activates via both RIP14 and RXR. Despite the very effective activation of RIP14 by tRA or TTNPB, relatively high concentrations of these compounds are required, and no evidence for direct binding of either compound was obtained using several approaches. These results suggest that RIP14 is the receptor for an as-yet-unidentified retinoid metabolite.
Six retinoid receptors have been thought to mediate the diverse effects of retinoids in both developing and adult animals (1, 2). Three types of retinoic acid receptors (RAR) (RARα, -β, and -γ) are activated by either all-trans-RA (tRA) or 9-cis-RA, and three types of retinoid X receptors (RXR) (RXRα, -β, and -γ) are activated specifically by 9-cis-RA (reviewed in ref. 3). Both the RARs and RXRs are members of the nuclear hormone receptor superfamily, which also includes the receptors for steroids, thyroid hormone, vitamin D3, and other ligands (4). In addition to these conventional receptors, this family contains a number of proteins that have no known ligands and are termed “orphan receptors.” Nuclear receptors exert their effects by binding to specific DNA sequences called “response elements” and by either positively or negatively regulating transcription. The RARs and many other members of the superfamily bind as heterodimers with RXR to response elements comprised of direct repeats of a distinct hexameric sequence (RGGTCA) or bind to palindromic (head-to-head) or everted palindromic (tail-to-tail) arrangements of this hexamer.
Recently, we used the yeast two hybrid system with RXR as “bait” to clone RXR interacting proteins (5). One of a number of proteins identified, RXR interacting protein 14 (RIP14), was an orphan receptor. RIP14 shows 82% amino acid sequence identity with the Drosophila ecdysone receptor (EcR) in the DNA binding domain, with ≈30–40% identity to other RXR heterodimer partners in the ligand binding domain. RIP14 is also the murine homolog of a rat orphan termed “FXR” (94% identity), which is reported to be activated by an unidentified metabolite of farnesol (6). In mice and rats, this orphan receptor is expressed primarily in the liver and kidney, with additional expression in gut and adrenal cortex (5, 6). As expected from the high degree of DNA binding domain similarity with the EcR, RIP14–RXR heterodimers also bind with high affinity to an EcR element (EcRE) from the Drosophila heat shock protein 27 (hsp27) promoter (5, 6).
Here we report that RIP14 can be specifically activated by either tRA or the synthetic retinoid [E]-4-[2-(5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-naphthalenyl)propen-1-yl]benzoic acid (TTNPB) previously thought to be a specific agonist for RARs (7, 8). Activation in the presence of either of these compounds is further augmented by addition of either 9-cis-RA or the RXR-specific agonist LG1069. Mutational analysis demonstrates that TTNPB activates the RIP14 moiety of the RIP14–RXR heterodimer, and the activation induced by 9-cis-RA and LG1069 is mediated by RXR. tRA activates both RIP14 and RXR, with RXR presumably being activated by metabolically produced 9-cis-RA (9). Despite the very effective activation of RIP14 by either tRA or TTNPB, dose response curves show a half maximal activation at 1–5 μM, and no protease resistance of RIP14 was observed in the presence of either compound. We conclude that RIP14 is the receptor for a yet-to-be-identified retinoid metabolite, joining the RARs and RXRs on the growing list of retinoid receptors.
MATERIALS AND METHODS
Transfections.
JEG-3 cells were maintained in DMEM plus 10% fetal calf serum (HyClone) at 37°C and 5% C02. Cells were plated into Falcon 12-well multi-well dishes (Becton Dickinson) in DMEM plus 10% charcoal stripped serum to ≈80% confluence, then the next day transfected with either 0.25 μg of CDM8 RIP14 or CDM8 RIP14 Δ19C and/or 0.025 μg of CDMh RXRα or CDM RXRα Δ19C plus enough CDM8 to bring the total amount of expression plasmid to 0.525 μg per well as indicated (5) plus 0.75 μg of hsp27–EcREx5 Δ mouse mammary tumor virus-luciferase (6) and 1 μg of TKGH per well using the diethylaminoethyl dextran/chloroquine method followed with dimethyl sulfoxide shock (10). After dimethyl sulfoxide shock, cells were maintained in DMEM plus 10% charcoal-stripped fetal bovine serum plus the indicated ligand or vehicle alone in the absence of ligand. TTNPB and 9-cis-RA were obtained from Biomol (Plymouth Meeting, PA), farnesol and tRA were from Sigma, and LG1069 was from Glaxo Wellcome (Research Park Triangle, NC) 48 h after transfection luciferase was assayed using the Promega Luciferase Assay System. Growth hormone was measured using the human growth hormone transient gene expression kit (Nichols Institute, San Juan Capistrano, CA).
Gel Mobility Shift Assay.
Gel mobility shift assays were performed as described (11). hRXRα and RARα in pT7lac, a histidine-tagged expression Escherichia coli expression vector, were expressed and purified as described (11). RIP14, RIP14 Δ9C, and RXRα Δ19C were expressed using the TNT T7-coupled reticulocyte lysate system (Promega). Products of the appropriate size in a parallel reaction containing 35S-methionine (New England Nuclear) were confirmed by SDS/PAGE analysis. The sequences of the top strand of the oligos used were as follows: hsp27, 5′-GATCTACAAGGGTTCAATGCACTTGTCCG; β2RAR element (β2RARE), 5′-GATCTGGGTAGGGTTCACCGAAAGTTCACTCGG; Slp HRE-3 GRE, 5′-GATCCAGAACAGCCTGTTCTA.
Plasmid Construction and Mutagenesis.
Standard methods for cloning were used (12). PCR (12) was used to delete amino acids 476–484 in RIP14–15 (5) using the primers 5′-GGAGAAGCTGCAGGAGC and 5′-AATTTAATTAGGCCAAAAGGGCATGCC and overlapping primers 5′-GTCCATCACGGGGTGAACTTGTGA and 5′-CACCCCGTGATGGACACCAGTGGG. The BspEI to SphI fragment was then subcloned into CDM8 RIP14–15 (referred to here as RIP14) to create RIP14 Δ9C. PCR was also used to delete amino acids 444–462 in hRXRα using the primers 5′-GCTGACGGAGCTTGTGTCC and 5′-CTCTGTAGGTAGTTGTCC and overlapping primers 5′-AGAGTCGCGGCCGCCTACCCGATGAG and 5′-TCTTCAAGCTCATCGGGTAGGCGGCC. The EagI fragment of RXRα was then subcloned into CDM RXRα to create CDM RXRΔ19C. Both subcloned fragments were sequenced in their entirety.
RESULTS
RIP14–RXR Heterodimers Are Specifically Activated by tRA, TTNPB, and 9-cis-RA.
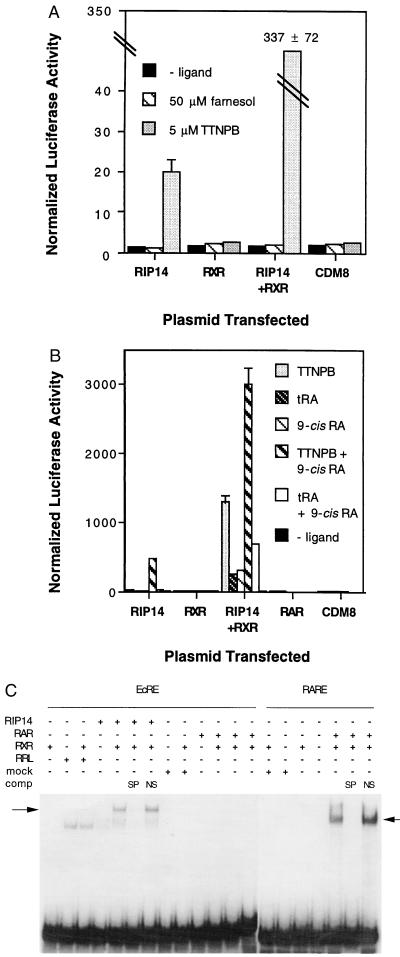
RIP14 is the mouse homolog of the rat orphan receptor FXR, which is reported to be activated by an unidentified farnesol metabolite (6). For reasons that remain unclear, we have not observed response to farnesol. However, in a screen of a number of potential farnesol metabolites and related compounds using a GAL4–RIP14 fusion analogous to those described for LXR (13), activation was observed with retinoids, particularly tRA and the synthetic retinoid TTNPB (data not shown). This response of the GAL4 fusion also was observed with the intact RIP14. As indicated in Fig. 1A, addition of 5 μM TTNPB to cells cotransfected with a RIP14 expression vector and a luciferase reporter containing five copies of the EcRE resulted in a 15-fold activation. The EcRE, which consists of an inverted repeat of the consensus receptor binding hexamer separated by 1 bp (IR-1), is bound by RIP14–RXR heterodimers (5) (see below), and addition of an RXR expression vector to the cotransfections substantially augmented the TTNPB response to ≈200-fold. In the same experiment, 50 μM farnesol was without effect. The response to TTNPB was observed in several cell lines, including JEG-3, CV-1, COSM6, and HepG2 (data not shown).
Figure 1.
RIP14 is specifically activated by retinoids. (A) RIP14 responds to TTNPB but not farnesol. JEG-3 cells were cotransfected with the indicated expression plasmids, hsp27 EcREx5 Δ mouse mammary tumor virus–luciferase, and a plasmid expressing hGH as an internal control and treated with no ligand, 50 μM farnesol, or 5 μM TTNPB. Luciferase values were normalized to the amount of growth hormone, and the mean ± SD of triplicate wells is graphed. This and all other transfection experiments presented are representative of results obtained in at least three separate experiments. (B) JEG-3 cells were cotransfected as described above and treated with no ligand, 5 μM TTNPB, 100 nM tRA, 1 μM 9-cis-RA, 5 μM TTNPB plus 1 μM 9-cis-RA, or 100 nM tRA plus 1 μM 9-cis-RA. In the same experiment, expression of functional RARβ was confirmed by cotransfection of pRSVRARβ or CDM8 with a β2RARE x3 TK luc (14) reporter plasmid. Normalized luciferase values of 458 ± 44 and 254 ± 14, respectively, were observed in the presence of 5 μM of TTNPB. (C) RIP14–RXR but not RAR–RXR heterodimers bind specifically to the hsp27 EcRE. As indicated, 9.4 fmol (15,000 cpm) of 32P-labeled hsp 27 (lanes 1–13, left to right) or β2RARE (lanes 14–20, left to right) were incubated with: 2 μl of in vitro-translated RIP14 or mock-translated reticulocyte lysate (RRL), 1 μl of E. coli expressed RXR or RARα or a mock preparation of E. coli alone (mock). SP, specific competitor (100 ng of the corresponding unlabeled oligonucleotide); NS, nonspecific competitor [100 ng of the unlabeled Slp HRE-3 GRE (15)]. A nonspecific complex was observed in the presence of reticulocyte lysate; specific complexes are indicated by arrows. The gels shown were run in parallel.
In further studies of activation of the RIP14–RXR complex by retinoids, tRA alone, 9-cis-RA alone as reported (6), or the combination of both retinoids induced a potent response from the EcRE reporter (Fig. 1B). Addition of 1 μM 9-cis-RA increased the response to 5 μM TTNPB, particularly in the absence of cotransfected RXR. Cotransfection of either RXR alone or the CDM8 vector alone did not confer responsiveness to any of these compounds. As expected, a reporter with a single copy of the EcRE showed a significant, but much weaker, response to TTNPB (2-fold) and to TTNPB plus 9-cis-RA (4-fold) in cotransfections with RIP14 or RIP14 plus RXR.
Important to note, neither the significant levels of RARs found in JEG-3 cells (see below) nor of cotransfected RARβ were able to transactivate the EcRE reporter in the presence of either TTNPB or tRA (Fig. 1B). In fact, addition of an RAR expression vector to cotransfections with either RIP14 alone or RIP14 plus RXR significantly inhibited TTNPB response (data not shown). In the same experiment, RAR did confer potent, retinoid-dependent transactivation of a reporter containing the RARE from the RARβ2 promoter (16, 17) (βRARE).
As expected from these and previous results, RIP14–RXR heterodimers bound specifically to the EcRE but neither RAR alone nor RAR–RXR heterodimers bound to the EcRE under conditions where RAR–RXR heterodimers bound efficiently to the βRARE (Fig. 1C). Further gel mobility shift analysis showed no additional complexes formed on the EcRE with the addition of RAR plus RIP14 or RAR plus RIP14 and RXR (data not shown). Based on both of these DNA binding studies and results of cotransfection experiments, we conclude that the observed responses cannot be attributed to activation of RARs by TTNPB, tRA, or 9-cis-RA.
AF-2 Deletion Mutations Confirm that RIP14 Is Responsive to TTNPB and tRA.
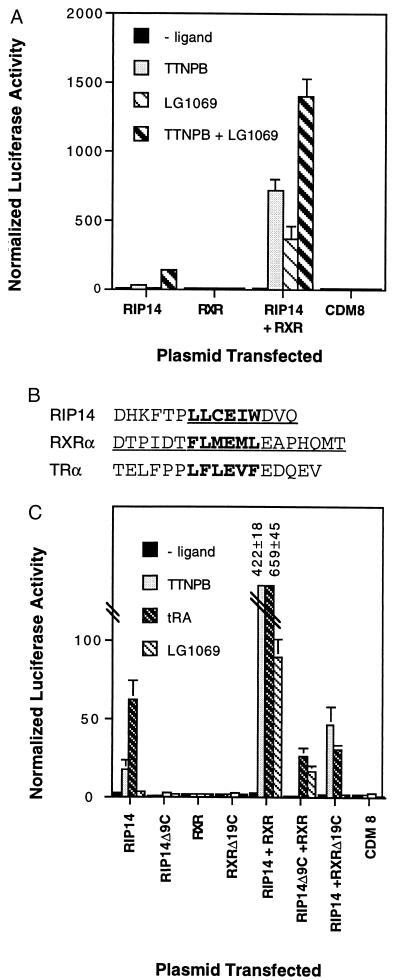
Both the stimulatory effect of RXR and the responsiveness of the combination of RIP14 plus RXR to 9-cis-RA are characteristic of results with heterodimeric complexes that are permissive for 9-cis-RA activation of RXR (18, 19). To determine the effect of specific activation of the RXR component of the RIP14–RXR heterodimer, the RXR-specific agonist LG1069 (8) was used. In transfection experiments carried out in the presence of both RIP14 and RXR, activation was observed with either 5 μM TTNPB or 1 μM LG1069 alone, and additional activation was observed when both were present (Fig. 2A). Because 1 μM of LG1069 saturates RXR response (8), these results are most consistent with a model in which RXR is specifically activated by LG1069 and RIP14 is specifically activated by TTNPB.
Figure 2.
Activation of RIP14 by TTNPB, RXR by LG1069, and both by tRA. (A) Cotransfected RIP14 plus RXR respond to both TTNPB and the RXR-specific agonist LG1069. JEG-3 cells were transfected as in Fig. 1 and treated with no ligand, 5 μM TTNPB, 1 μM LG1069, or 5 μM TTNPB plus 1 μM LG1069. (B) AF-2 regions deleted from RIP14 and RXRα. Amino acids 476–484 of RIP14 and 444–462 of RXRα were deleted to generate RIP14–Δ9C and RXR–Δ19C, respectively. The regions corresponding to the AF-2 motif (36) are identified in bold, and the regions deleted in each receptor are underlined. TRα is included for comparison. (C) AF-2 deletions demonstrate that RIP14 is activated by TTNPB, that RXR is activated by LG1069, and that both receptors respond to tRA. JEG-3 cells were transfected with the indicated expression vectors as in Fig. 1 and treated with either no ligand, 5 μM TTNPB, 10 μM tRA, or 100 nM LG1069.
To confirm and extend this conclusion, the AF-2 domains of RIP14 and RXR were deleted. Deletion of the last 19 amino acids generated RXR Δ19C (Fig. 2B), which has been demonstrated to be unresponsive to RXR-specific ligands but to retain both heterodimerization function and ligand activation of heterodimer partners (21). As expected, this mutant receptor was unable to activate a reporter construct containing five copies of the CRBPII RXR response element (22) in the presence of 1 μM of LG1069, unlike wild-type RXR (data not shown). A similar 9-amino acid mutation was introduced into RIP14, creating RIP14 Δ9C (Fig. 2B). If RIP14 is the target for TTNPB activation and RXR is the target for LG1069 activation, TTNPB activation should be lost with RIP14–Δ9C and RXR Δ19C should lose LG1069 signaling. This is exactly what was observed (Fig. 2C). In both cases, response associated with the mutated receptor was completely absent, and that associated with its partner was maintained, albeit at a decreased level. This decrease is consistent with several previous reports (21, 23–25) and presumably reflects a contribution by the unliganded AF-2 domain to the overall activation associated with the ligand-bound partner.
Interpretation of the responses of the AF-2 mutants to tRA is somewhat complicated by the conversion of tRA to 9-cis-RA (9). Thus, 10 μM of tRA resulted in 18-fold induction in cotransfection experiments with RIP14 plus RXR Δ19C, indicating that RIP14 is activated by tRA. However, the combination of RIP14 Δ9C plus RXR also was induced 20-fold, indicating that both partners are responsive to high concentrations of tRA (Fig. 2C). The combination of RIP14 + RXRΔ19C is stimulated by tRA but is completely unresponsive to the RXR-specific ligand LG1069, and RIP14 alone is substantially activated by tRA yet not LG1069, so we concluded that the response of RIP14–RXR heterodimers to tRA is at least partially due to activation of RIP14.
To confirm that the loss of response of RXRΔ19C to LG1069 and RIP14Δ9C to TTNPB was not caused by a decreased expression or instability of the mutants, they were cotransfected with both wild-type RIP14 and RXR. In the presence of an equimolar amount of RIP14 Δ9C expression vector, RIP14–RXR response to TTNPB was decreased by 65%. In the presence of an equal amount of RXR Δ19C expression vector, RIP14–RXR response to LG1069 was only 8% that of RIP14–RXR (data not shown). These dominant negative effects are expected from previous results with other receptors and confirm the expression of the mutants. Gel mobility shift assays using in vitro-translated mutant receptors and wild-type RIP14 [and E. coli-expressed, wild-type RXR (similar to those shown in Fig. 1C)] also confirmed that both mutants bound to the EcRE with a similar affinity to wild-type RIP14–RXR heterodimers (data not shown). Overall, these results demonstrate that RIP14–RXR heterodimers belong to the growing class that is permissive for RXR signaling (18). More importantly, we conclude that RIP14 is the target of activation by TTNPB and tRA.
Neither TTNPB nor tRA Act as Direct Ligands for RIP14.
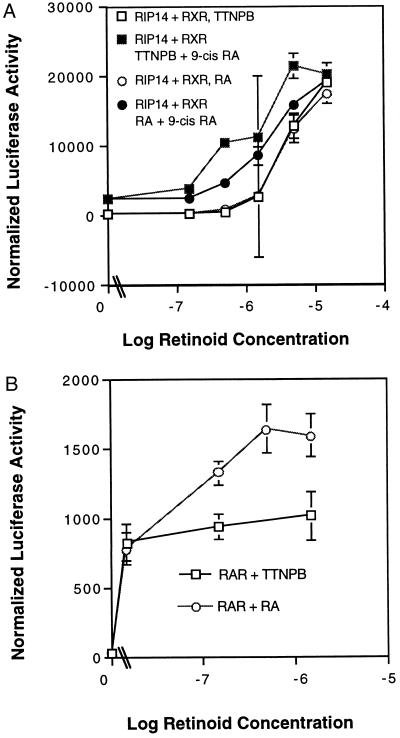
TTNPB is a high affinity RAR agonist (7, 26). A dose response was carried out to determine the EC50 for TTNPB-dependent transactivation of RIP14 in the absence and presence of 9-cis-RA (Fig. 3A). In the absence of 9-cis-RA, the EC50 for activation of cotransfected RIP14 plus RXR by TTNPB is greater than 1 μM and is ≈0.5–1 μM in the presence of 9-cis-RA. This apparent affinity is ≈100-fold less than that observed for endogenous RAR activation by TTNPB from an RARE-containing reporter in the same experiment (Fig. 3B). The high concentration of TTNPB required for RIP14 activation provides an additional confirmation that endogenous RARs are not involved in this process. It also suggests that TTNPB, despite its ability to strongly activate RIP14–RXR heterodimers, is not a high affinity RIP14 ligand. A similar dose response was obtained with tRA, with an EC50 of ≈5 μM (Fig. 3A), that was slightly decreased in the presence of 9-cis-RA. Thus, tRA is also not a high affinity RIP14 ligand (Fig. 3A).
Figure 3.
Dose response of RIP14 activation by retinoids. (A) JEG-3 cells were transfected as in Fig. 1 with RIP14, RXR, and hsp27–EcREx5 Δ mouse mammary tumor virus–luciferase and treated with 0–10 μM TTNPB ± 1 μM 9-cis-RA and 0–10 μM tRA ± 1 μM 9-cis-RA. (B) JEG-3 cells were transfected with CDM8 as a carrier plasmid and the β2RARE x3 TK luc reporter plasmid, and the response of endogenous RAR to either 0–1 μM TTNPB or tRA was determined.
The time courses of activation of RIP14 and RAR were compared to explore whether metabolism of TTNPB and tRA is required for activation of RIP14 (Table 1). After 3 h of treatment, the first time point at which significant response is observed, the activation of the endogenous RARs by TTNPB or tRA was 5.7% or 6.3% of that observed at 24 h, respectively. In contrast, the activation of RIP14 plus RXR with TTNPB or tRA is only 1.3% or 2.8% of that at 24 h. This relative lag in the activation of RIP14 plus RXR is also evident at 6 h, particularly for tRA. These results are consistent with the hypothesis that both compounds must be metabolized before activation of RIP14–RXR heterodimers can occur.
Table 1.
Time course of RIP14 activation by TTNPB and tRA
| h
|
|||||
|---|---|---|---|---|---|
| 0 | 1 | 3 | 6 | 24 | |
| RIP14 + RXR | |||||
| + 5 μM TTNPB | 0.2 ± 0.0 | 0.2 ± 0.1 | 1.3 ± 0.5 | 10.4 ± 1.0 | 100.0 ± 0.0 |
| + 5 μM tRA | 0.3 ± 0.0 | 0.3 ± 0.0 | 2.8 ± 2.6 | 3.8 ± 0.5 | 100.0 ± 0.0 |
| Endogenous RAR | |||||
| + 5 μM TTNPB | 0.4 ± 0.2 | 0.4 ± 0.2 | 5.7 ± 2.0 | 21.5 ± 4.8 | 100.0 ± 0.0 |
| + 5 μM tRA | 0.4 ± 0.0 | 0.6 ± 0.1 | 6.3 ± 1.0 | 54.1 ± 12.7 | 100.0 ± 0.0 |
JEG-3 cells were cotransfected as in Fig. 1 with either wild-type RIP14 plus RXR and hsp27 EcREx5 ΔMTV–luciferase or CDM 8 and β2RARE x3 TK luc reporter; 24 h after transfection, the cells were treated with either 5 μM TTNPB or 5 μM tRA and harvested at the indicated time point. The luciferase activity at the indicated time points is presented by comparison to that observed at 24 h and is the average of three separate experiments. For RIP14 plus RXR, the relative activation at 24 h was 530- ± 110-fold with TTNPB and 340- ± 46-fold with tRA. For the endogenous RARs, the relative activation at 24 h was 160- ± 45-fold with TTNPB and 270- ± 34-fold with tRA.
Two independent approaches were taken to test the possibility that either TTNPB or tRA is a low affinity ligand for RIP14. The first is the protease sensitivity assay. Ligand binding stabilizes a number of receptors, including RARs, TR, and RXRs, against protease cleavage (27, 28). Several different proteases (chymotrypsin, trypsin, and V8 protease) were tested with RIP14 under conditions in which RARs showed significant protection in the presence of TTNPB or tRA. However, no protection of RIP14 was observed with either compound, in the presence or absence of 9-cis-RA, RXR, or both (data not shown). As a second approach, the interaction of RIP14 with proteins that show ligand-dependent interaction with other known receptors was examined using the yeast two-hybrid system. In this system, Trip1 shows strong, ligand-dependent interactions with TR, RXR, and several other superfamily members (29, 30). Although a LexA–RIP14 fusion did show the expected interaction with an appropriate RXR fusion, it showed no interaction with a Trip1 fusion, in either the presence or absence of TTNPB or tRA. This is not due to a general retinoid impermeability of this yeast strain (ref. 31 and data not shown).
DISCUSSION
FXR, the rat homolog of RIP14, was reported to be activated in response to high concentrations of farnesol, and this response was assumed to be a consequence of an unidentified farnesol metabolite (6). Despite the use of the same response element and same CV-1 cell line where the original activation was described, we have not observed such response with RIP14. This could be a consequence of a difference between the rat and murine receptors, although their high degree of amino acid sequence identity makes this seem unlikely. Particularly because farnesol, as an terpenoid or isoprenoid compound, is related to retinoids, it also seemed possible that differences in farnesol metabolism in the transfected cells could account for the discrepancy and that the proximal activating compound could be a retinoid or retinoid-related compound. In support of this possibility, we have found that the RIP14 component of RIP14–RXR heterodimers is specifically activated by retinoids.
The activation of RIP14 by tRA and TTNPB does not involve RARs because (i) RARs have no effect on the IR-1 reporter transactivated by RIP14, (ii) RARs do not bind the IR-1 response element, either alone or in the presence of RXR or RIP14; and (iii) the concentration of tRA or TTNPB required is much higher than that required for activation of RARs. RXR does not mediate the TTNPB responsiveness observed in the presence of RIP14 on the EcRE: (i) TTNPB does not bind or activate RXRs (11), (ii) mutation of the RXR AF-2 domain blocks response to the RXR-specific agonist LG1069 but does not block TTNPB response, and (iii) significant TTNPB response is observed even when the RXR component is activated by saturating doses of LG1069. This latter argument for RXR also supports the conclusion that RIP14 is the direct target for TTNPB activation, which is confirmed by the observations that (i) cotransfection with RIP14 is required for transactivation of the IR-1 element, (ii) RIP14–RXR heterodimers bind that element with high affinity, and (iii) mutation of the RIP14 AF-2 domain completely blocks TTNPB response.
The activation of RIP14 by retinoids requires concentrations that are well above those necessary to activate the RARs. Recent results with other receptors suggest that oxysterols and fatty acids may act as true ligands at comparable or even higher concentrations (13, 32). However, we have found no evidence for direct binding of either tRA or TTNPB to RIP14. tRA has been shown to undergo extensive cellular metabolism (9), and recent work has shown that TTNPB can also be metabolized to several compounds (34). Particularly because the time course of activation indicates a lag before the response of RIP14 to either compound, it is possible that the proximal activator of RIP14 is a retinoid metabolite. Alternatively, it remains possible that additional approaches will reveal a direct, low affinity interaction between RIP14 and tRA or TTNPB or even that the physiological RIP14 ligand will prove to be neither a retinoid nor a farnesoid but a related terpenoid compound.
Retinoid responses have been assumed to be mediated by the three RARs and the three RXRs. These two classes of receptors have quite divergent ligand binding domains, showing only ≈30% amino acid sequence identity in pairwise comparisons. RIP14 is approximately as closely related to either the RARs or the RXRs as they are to each other, sharing 31% sequence identity with RARα and 27% sequence identity with RXRα. The assignment of retinoid response to the RARs and RXRs is strongly supported by the large number of developmental abnormalities associated with vitamin A deficiency that are reproduced in animals with RAR or RXR gene deficiencies, particularly animals with more than one defective gene (2). Nonetheless, the phenotypes of the mutant animals seem milder than what might have been expected, and it is possible that that superfamily members beyond the known retinoid receptors contribute to retinoid signaling. There are several mechanisms by which orphans or other conventional receptors could make such contributions. One is the 9-cis-RA responsiveness of a subset of heterodimer complexes (18, 19). The RXR component of the RIP14–RXR heterodimers is responsive to activation by RXR-specific ligands, so RIP14 joins LXR, NGFI-B, and the peroxisome proliferator-activated receptors as a member of this subset. A second mechanism is the ability of several orphan receptors to bind and transactivate RAREs in the absence of retinoids. HNF-4, for example, can transactivate RAREs of the DR-1 type in the absence of retinoids (35, 36), and MB67, an orphan receptor isolated in this laboratory, can similarly transactivate DR-5 RAREs (14). These and other orphans may direct basal, retinoid-independent levels of expression of retinoid target genes and could therefore blunt the effects of RAR loss in appropriate tissues.
The activation of RIP14 by retinoids as described here clearly raises a third possibility: that there are additional retinoid receptors. RIP14 is predominantly expressed in liver and kidney, with additional expression detected in gut and adrenal medulla (5, 6), and we suggest that it plays an important role in retinoid signaling in those tissues. The identification of its physiological ligand should allow a more direct determination of that role.
Acknowledgments
We thank Drs. Ron Evans and Barry Forman for the hsp27 EcREx5 Δ mouse mammary tumor virus-luciferase construct. A.M.Z. is supported by National Research Service Award F32 DK09641 from the National Institute of Diabetes and Digestive and Kidney Diseases. W.S. is supported by a fellowship from the Helen Hay Whitney Foundation.
ABBREVIATIONS
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- RIP14
RXR interacting protein 14
- tRA
all-trans-RA
- hsp
heat shock protein
- TTNPB
[E]-4-[2-(5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-naphthalenyl)propen-1-yl]benzoic acid
- LG1069
4-[1-(3, 5, 5, 8, 8-pentamethyl-5, 6, 7, 8-tetrahydro-2-naphthyl)ethenyl]benzoic acid
- β2RARE
β2RAR element
- EcRE
ecdysone receptor response element
References
- 1.Means A L, Gudas L J. Annu Rev Biochem. 1995;64:201–233. doi: 10.1146/annurev.bi.64.070195.001221. [DOI] [PubMed] [Google Scholar]
- 2.Kastner P, Mark M, Chambon P. Cell. 1995;83:859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- 3.Leid M, Kastner P, Chambon P. Trends Biochem Sci. 1992;17:427–433. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]
- 4.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans R M. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seol W, Choi H-S, Moore D D. Mol Endocrinol. 1995;9:72–85. doi: 10.1210/mend.9.1.7760852. [DOI] [PubMed] [Google Scholar]
- 6.Forman B M, Goode E, Chen J, Oro A E, Bradley D J, Perlmann T, Noonan D J, Burka L T, McMorris T, Lamph W W, Evans R M, Weinberger C W. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 7.Crettaz M, Baron A, Siegenthaler G, Hunziker W. Biochem J. 1990;272:391–397. doi: 10.1042/bj2720391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boehm M F, Zhang L, Badea B A, White S K, Mais D E, Berger E, Suto C M, Goldman M E, Heyman R A. J Med Chem. 1994;37:2930–2941. doi: 10.1021/jm00044a014. [DOI] [PubMed] [Google Scholar]
- 9.Heyman R A, Mangelsdorf D J, Dyck J A, Stein R B, Eichele G, Evans R M, Thaller C. Cell. 1992;68:379–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- 10.Seol W, Mahon M J, Lee Y K, Moore D D. Mol Endocrinol. 1996;10:1646–1655. doi: 10.1210/mend.10.12.8961273. [DOI] [PubMed] [Google Scholar]
- 11.Zavacki A M, Harney J W, Brent G A, Larsen P R. Endocrinology. 1996;137:2833–2841. doi: 10.1210/endo.137.7.8770904. [DOI] [PubMed] [Google Scholar]
- 12.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1997. [Google Scholar]
- 13.Lehmann J M, Kliewer S A, Moore L B, Smith-Oliver T A, Oliver B B, Su J L, Sundseth S S, Winegar D A, Blanchard D E, Spencer T A, Willson T M. J Biol Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 14.Baes M, Gulick T, Choi H-S, Martinoli M G, Simha D, Moore D D. Mol Cell Biol. 1994;14:1544–1552. doi: 10.1128/mcb.14.3.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler A J, Danielsen M, Robins D M. Proc Natl Acad Sci USA. 1992;89:11660–11663. doi: 10.1073/pnas.89.24.11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sucov H M, Murakami K K, Evans R M. Proc Natl Acad Sci USA. 1990;87:5392–5396. doi: 10.1073/pnas.87.14.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de The H, Vivanco-Ruiz M, Tiollais P, Stunnenberg H, Dejean A. Nature (London) 1990;343:177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- 18.Mangelsdorf D J, Evans R M. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 19.Leblanc B P, Stunnenberg H G. Genes Dev. 1995;9:1811–1816. doi: 10.1101/gad.9.15.1811. [DOI] [PubMed] [Google Scholar]
- 20.Barettino D, Vivanco-Ruiz M M, Stunnenberg H G. EMBO J. 1994;13:3039–3049. doi: 10.1002/j.1460-2075.1994.tb06603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X-K, Salbert G, Lee M-O, Pfahl M. Mol Cell Biol. 1994;14:4311–4323. doi: 10.1128/mcb.14.6.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangelsdorf D J, Umesono K, Kliewer S A, Borgmeyer U, Ong E S, Evans R M. Cell. 1991;66:555–561. doi: 10.1016/0092-8674(81)90018-0. [DOI] [PubMed] [Google Scholar]
- 23.Leng X, Blanco J, Tsai S Y, Ozato K, O’Malley B W, Tsai M J. J Biol Chem. 1994;269:31436–31442. [PubMed] [Google Scholar]
- 24.Schulman I G, Juguilon H, Evans R M. Mol Cell Biol. 1996;16:3807–3813. doi: 10.1128/mcb.16.7.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Botling J, Castro D S, Öberg F, Nilsson K, Perlmann T. J Biol Chem. 1997;272:9443–9449. doi: 10.1074/jbc.272.14.9443. [DOI] [PubMed] [Google Scholar]
- 26.Strickland S, Breitman T R, Frickel F, Nurrenbach A, Hadicke E, Sporn M B. Cancer Res. 1983;43:5268–5272. [PubMed] [Google Scholar]
- 27.Leng X, Tsai S-Y, O’Malley B W, Tsai M J. J Steroid Biochem Mol Biol. 1993;46:643–661. doi: 10.1016/0960-0760(93)90306-h. [DOI] [PubMed] [Google Scholar]
- 28.Leng X, Blanco J, Tsai S Y, Ozato K, O’Malley B W, Tsai M J. Mol Cell Biol. 1995;15:255–263. doi: 10.1128/mcb.15.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J W, Ryan F, Swaffield J C, Johnston S A, Moore D D. Nature (London) 1995;374:91–94. doi: 10.1038/374091a0. [DOI] [PubMed] [Google Scholar]
- 30.Lee J W, Choi H-S, Gyuris J, Brent R, Moore D D. Mol Endocrinol. 1995;9:243–254. doi: 10.1210/mend.9.2.7776974. [DOI] [PubMed] [Google Scholar]
- 31.Lee J W, Moore D D, Heyman R A. Mol Endocrinol. 1994;8:1245–1253. doi: 10.1210/mend.8.9.7838157. [DOI] [PubMed] [Google Scholar]
- 32.Janowski B A, Willy P J, Devi T R, Falck J R, Mangelsdorf D J. Nature (London) 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 33.Kliewer S, Sundseth S, Jones S, Brown P, Wisely G, Koble C, Devchann P, Wahli W, Willson T, Lenhard J, Lehmann J. Proc Natl Acad Sci USA. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pignatello M A, Kauffman F C, Levin A A. Toxicol Appl Pharmacol. 1997;142:319–327. doi: 10.1006/taap.1996.8047. [DOI] [PubMed] [Google Scholar]
- 35.Mietus-Snyder M, Sladek F M, Ginsburg G S, Kuo C-F, Ladias J A, Darnell J E, Jr, Karathanasis S K. Mol Cell Biol. 1992;12:1708–1718. doi: 10.1128/mcb.12.4.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ladias J A A, Hadzopoulou-Cladaras M, Kardassis D, Cardot P, Cheng J, Zannis V, Cladaras C. J Biol Chem. 1992;267:15849–15860. [PubMed] [Google Scholar]