Abstract
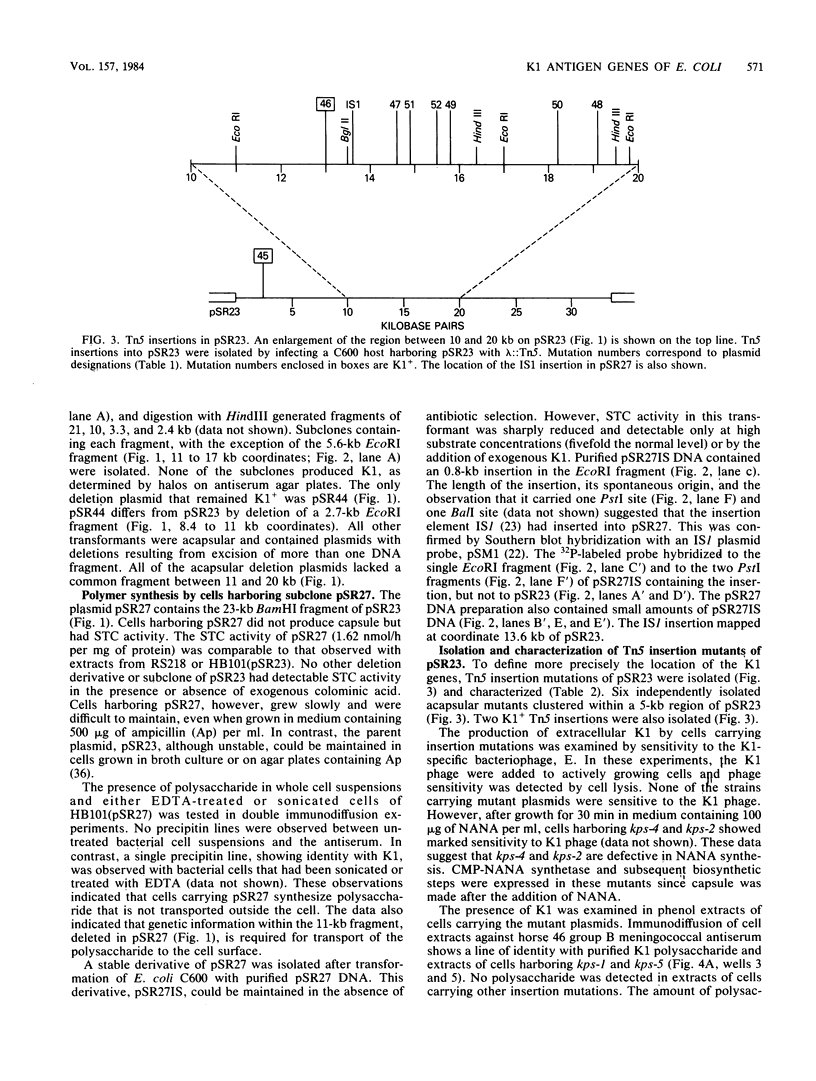
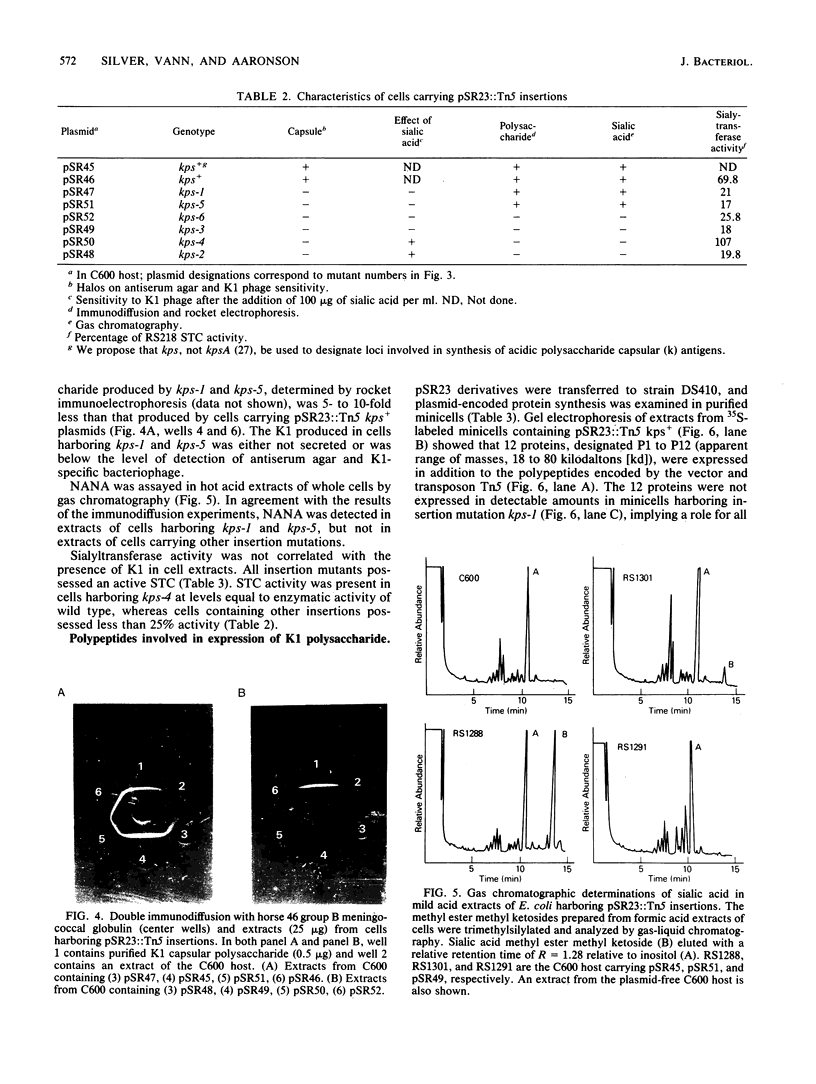
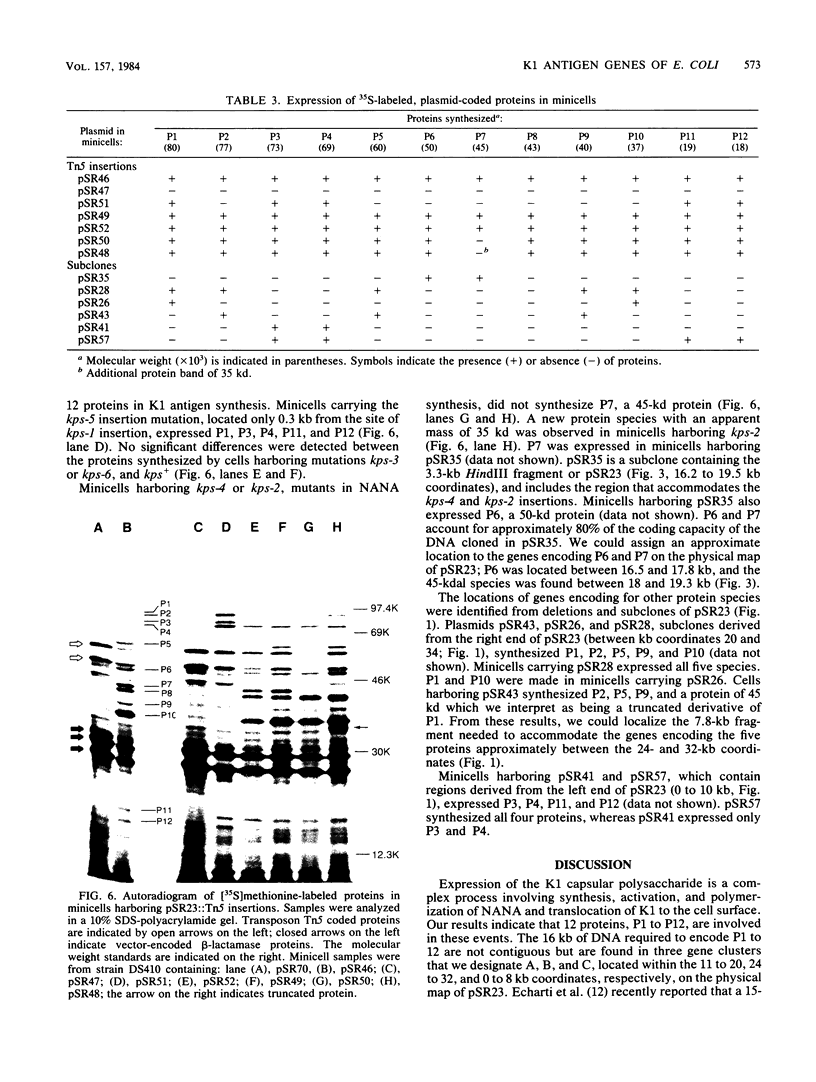
The plasmid pSR23, composed of a 34-kilobase E. coli chromosomal fragment inserted into the BamHI site of the pHC79 cosmid cloning vector, contains genes encoding biosynthesis of the K1 capsular polysaccharide. Deletions, subclones, and Tn5 insertion mutants were used to localize the K1 genes on pSR23. The only deletion derivative of pSR23 that retained the K1 phenotype lacked a 2.7-kilobase EcoRI fragment. Subclones containing HindIII and EcoRI fragments of pSR23 did not produce K1. Cells harboring pSR27, a subclone containing a 23-kilobase BamHI fragment, synthesized K1 that was not detectable extracellularly. Six acapsular Tn5 insertion mutants of three phenotypic classes were observed. Class I mutants synthesized K1 only when N-acetylneuraminic acid (NANA) was provided in the medium. Reduced amounts of K1 were detectable in cell extracts of class II mutants. Class III mutants did not produce detectable K1 in either extracts or when cells were provided exogenous NANA. All mutants had sialyltransferase activity. Analysis in the E. coli minicell system of proteins expressed by derivatives of pSR23 identified a minimum of 12 polypeptides, ranging in size from 18,000 to 80,000 daltons, involved in K1 biosynthesis. The 16-kilobase coding capacity required for the proteins was located in three gene clusters designated A, B, and C. We propose that the A cluster contains a NANA operon of two genes that code for proteins with apparent molecular weights of 45,000 and 50,000. The A region also includes a 2-kilobase segment involved in regulation of K1 synthesis. The B region encoding five protein species appears responsible for the translocation of the polymer from its site of synthesis on the cytoplasmic membrane to the cell surface. The C region encodes four protein species. Since the three gene clusters appear to be coordinately regulated. we propose that they constitute a kps regulon.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Manning P. A., Edelbluth C., Herrlich P. Export without proteolytic processing of inner and outer membrane proteins encoded by F sex factor tra cistrons in Escherichia coli minicells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4837–4841. doi: 10.1073/pnas.76.10.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerswald E. A., Ludwig G., Schaller H. Structural analysis of Tn5. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):107–113. doi: 10.1101/sqb.1981.045.01.019. [DOI] [PubMed] [Google Scholar]
- BARRY G. T. Colominic acid, a polymer of N-acetylneuraminic acid. J Exp Med. 1958 Apr 1;107(4):507–521. doi: 10.1084/jem.107.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Weiss A., Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980 May;142(2):439–446. doi: 10.1128/jb.142.2.439-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Brodribb A. J. Treatment of symptomatic diverticular disease with a high-fibre diet. Lancet. 1977 Mar 26;1(8013):664–666. doi: 10.1016/s0140-6736(77)92112-2. [DOI] [PubMed] [Google Scholar]
- COMB D. G., ROSEMAN S. The sialic acids. I. The structure and enzymatic synthesis of N-acetylneuraminic acid. J Biol Chem. 1960 Sep;235:2529–2537. [PubMed] [Google Scholar]
- DEWITT C. W., ROWE J. A. Sialic acids (N,7-O-diacetylneuraminic acid and N-acetylneuraminic acid) in Escherichia coli. I. Isolation and identification. J Bacteriol. 1961 Dec;82:838–848. doi: 10.1128/jb.82.6.838-848.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Echarti C., Hirschel B., Boulnois G. J., Varley J. M., Waldvogel F., Timmis K. N. Cloning and analysis of the K1 capsule biosynthesis genes of Escherichia coli: lack of homology with Neisseria meningitidis group B DNA sequences. Infect Immun. 1983 Jul;41(1):54–60. doi: 10.1128/iai.41.1.54-60.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHOSH S., ROSEMAN S. THE SIALIC ACIDS. IV. N-ACYL--D-GLUCOSAMINE 6-PHOSPHATE 2-EPIMERASE. J Biol Chem. 1965 Apr;240:1525–1530. [PubMed] [Google Scholar]
- Gotschlich E. C., Fraser B. A., Nishimura O., Robbins J. B., Liu T. Y. Lipid on capsular polysaccharides of gram-negative bacteria. J Biol Chem. 1981 Sep 10;256(17):8915–8921. [PubMed] [Google Scholar]
- Gross R. J., Cheasty T., Rowe B. Isolation of bacteriophages specific for the K1 polysaccharide antigen of Escherichia coli. J Clin Microbiol. 1977 Dec;6(6):548–550. doi: 10.1128/jcm.6.6.548-550.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Kanegasaki S., Jann K. Demonstration by membrane reconstitution of a butanol-soluble intermediate in the biosynthesis of the O9 antigen of Escherichia coli. Eur J Biochem. 1979 Apr 2;95(2):287–293. doi: 10.1111/j.1432-1033.1979.tb12964.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeBlanc D. J., Lee L. N. Characterization of two tetracycline resistance determinants in Streptococcus faecalis JH1. J Bacteriol. 1982 May;150(2):835–843. doi: 10.1128/jb.150.2.835-843.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickel S., Bauer W. Isolation, by tetracycline selection, of small plasmids derived from R-factor R12 in Escherichia coli K-12. J Bacteriol. 1976 Jul;127(1):644–655. doi: 10.1128/jb.127.1.644-655.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov F., Orskov I., Sutton A., Schneerson R., Lin W., Egan W., Hoff G. E., Robbins J. B. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J Exp Med. 1979 Mar 1;149(3):669–685. doi: 10.1084/jem.149.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Nyman K. Genetic mapping of the antigenic determinants of two polysaccharide K antigens, K10 and K54, in Escherichia coli. J Bacteriol. 1974 Oct;120(1):43–51. doi: 10.1128/jb.120.1.43-51.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Sharma V., Orskov F. Genetic mapping of the K1 and K4 antigens (L) of Escherichia coli. Non-allelism of K(L) antigens with K antigens of O8:K27(A), O8:K8(L) and O9:K57(B). Acta Pathol Microbiol Scand B. 1976 Jun;84(3):125–131. [PubMed] [Google Scholar]
- Paakkanen J., Gotschlich E. C., Mäkelä P. H. Protein K: a new major outer membrane protein found in encapsulated Escherichia coli. J Bacteriol. 1979 Sep;139(3):835–841. doi: 10.1128/jb.139.3.835-841.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Rohr T. E., Troy F. A. Structure and biosynthesis of surface polymers containing polysialic acid in Escherichia coli. J Biol Chem. 1980 Mar 25;255(6):2332–2342. [PubMed] [Google Scholar]
- Rothstein S. J., Jorgensen R. A., Yin J. C., Yong-di Z., Johnson R. C., Reznikoff W. S. Genetic organization of Tn5. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):99–105. doi: 10.1101/sqb.1981.045.01.018. [DOI] [PubMed] [Google Scholar]
- Schiffer M. S., Oliveira E., Glode M. P., McCracken G. H., Jr, Sarff L. M., Robbins J. B. A review: relation between invasiveness and the K1 capsular polysaccharide of Escherichia coli. Pediatr Res. 1976 Feb;10(2):82–87. doi: 10.1203/00006450-197602000-00002. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Aaronson W., Sutton A., Schneerson R. Comparative analysis of plasmids and some metabolic characteristics of Escherichia coli K1 from diseased and healthy individuals. Infect Immun. 1980 Jul;29(1):200–206. doi: 10.1128/iai.29.1.200-206.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. P., Finn C. W., Vann W. F., Aaronson W., Schneerson R., Kretschmer P. J., Garon C. F. Molecular cloning of the K1 capsular polysaccharide genes of E. coli. Nature. 1981 Feb 19;289(5799):696–698. doi: 10.1038/289696b0. [DOI] [PubMed] [Google Scholar]
- Troy F. A., McCloskey M. A. Role of a membranous sialyltransferase complex in the synthesis of surface polymers containing polysialic acid in Escherichia coli. Temperature-induced alteration in the assembly process. J Biol Chem. 1979 Aug 10;254(15):7377–7387. [PubMed] [Google Scholar]
- Troy F. A., Vijay I. K., McCloskey M. A., Rohr T. E. Synthesis of capsular polymers containing polysialic acid in Escherichia coli 07-K1. Methods Enzymol. 1982;83:540–548. doi: 10.1016/0076-6879(82)83050-4. [DOI] [PubMed] [Google Scholar]
- Troy F. A., Vijay I. K., Tesche N. Role of undecaprenyl phosphate in synthesis of polymers containing sialic acid in Escherichia coli. J Biol Chem. 1975 Jan 10;250(1):156–163. [PubMed] [Google Scholar]
- Weeke B. Rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:37–46. doi: 10.1111/j.1365-3083.1973.tb03777.x. [DOI] [PubMed] [Google Scholar]