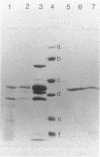
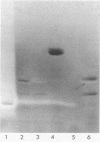
Abstract
Nocardia brasiliensis possess proteolytic activities that can be readily detected in a variety of media. In a modified formulation of a growth medium originally used for Streptomyces aureofaciens, N. brasiliensis was found to secrete proteolytic enzymes, one of which was capable of hydrolyzing casein. This enzyme was purified to homogeneity from cell-free culture filtrates of N. brasiliensis. The purification procedure included ion-exchange chromatography on carboxymethyl-Sepharose, gel filtration on Sephadex G-100, and affinity chromatography, using a hemoglobin-Sepharose resin. The molecular weight of the N. brasiliensis protease was found to be 25,000 by gel filtration and 35,000 by sodium dodecyl sulfate-discontinuous gel electrophoresis. The enzyme is inhibited by o-phenanthroline and 8-hydroxyquinoline-5-sulfonic acid but is not affected by EDTA. Average values for its kinetic parameters were 0.288 mumol of hemoglobin solubilized per min per mg of enzyme for Vmax and 0.76 mM for Km, using hemoglobin as the substrate.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua G. K., Bushuk W. Purification of wheat proteases by affinity chromatography on hemoglobin-Sepharose column. Biochem Biophys Res Commun. 1969 Oct 22;37(3):545–550. doi: 10.1016/0006-291x(69)90950-4. [DOI] [PubMed] [Google Scholar]
- GALLOP P. M., SEIFTER S., MEILMAN E. Studies on collagen. I. The partial purification, assay, and mode of activation of bacterial collagenase. J Biol Chem. 1957 Aug;227(2):891–906. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacks S. A., Springhorn S. S. Renaturation of enzymes after polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate. J Biol Chem. 1980 Aug 10;255(15):7467–7473. [PubMed] [Google Scholar]
- Laluce C., Molinari R. Selection of a chemically defined medium for submerged cultivation of Streptomyces aureofaciens with high extracellular caseinolytic activity. Biotechnol Bioeng. 1977 Dec;19(12):1863–1884. doi: 10.1002/bit.260191210. [DOI] [PubMed] [Google Scholar]
- MORIHARA K. PRODUCTION OF ELASTASE AND PROTEINASE BY PSEUDOMONAS AERUGINOSA. J Bacteriol. 1964 Sep;88:745–757. doi: 10.1128/jb.88.3.745-757.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihara K. Comparative specificity of microbial proteinases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):179–243. doi: 10.1002/9780470122860.ch5. [DOI] [PubMed] [Google Scholar]
- Morihara K., Oka T., Tsuzuki H. The compound active site of Bacillus subtilis neutral protease: some properties of six subsites. Arch Biochem Biophys. 1969 Jul;132(2):489–501. doi: 10.1016/0003-9861(69)90393-2. [DOI] [PubMed] [Google Scholar]
- Narahashi Y., Yanagita M. Studies on proteolytic enzymes (pronase) of Streptomyces griseus K-1. I. Nature and properties of the proteolytic enzyme system. J Biochem. 1967 Dec;62(6):633–641. doi: 10.1093/oxfordjournals.jbchem.a128718. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Trop M., Birk Y. The specificity of proteinases from Streptomyces griseus (pronase). Biochem J. 1970 Jan;116(1):19–25. doi: 10.1042/bj1160019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wählby S. Studies on Streptomyces griseus protease. I. Separation of DFP-reacting enzymes and purification of one of the enzymes. Biochim Biophys Acta. 1968 Feb 5;151(2):394–401. doi: 10.1016/0005-2744(68)90106-x. [DOI] [PubMed] [Google Scholar]