Abstract
Cardiac muscarinic receptors activate an inwardly rectifying K+ channel, IK+Ach, via pertussis toxin (PT)-sensitive heterotrimeric G proteins (in heart Gi2, Gi3, or Go). We have used embryonic stem cell (ES cell)-derived cardiocytes with targeted inactivations of specific PT-sensitive α subunits to determine which G proteins are required for receptor-mediated regulation of IK+Ach in intact cells. The muscarinic agonist carbachol increased IK+Ach activity in ES cell-derived cardiocytes from wild-type cells, in cells lacking αo, and in cells lacking the PT-insensitive G protein αq. In cells with targeted inactivation of αi2 or αi3, channel activation by both carbachol and adenosine was blocked. Carbachol-induced channel activation was restored in the αi2- and αi3-null cells by reexpressing the previously targeted gene and guanosine 5′-[γ-thio] triphosphate was able to fully activate IK+Ach in excised membranes patches from these mutants. In contrast, negative chronotropic responses to both carbachol and adenosine were preserved in cells lacking αi2 or αi3. Our results show that expression of two specific PT-sensitive α subunits (αi2 and αi3 but not αo) is required for normal agonist-dependent activation of IK+Ach and suggest that both αi2- and αi3-containing heterotrimeric G proteins may be involved in the signaling process. Also the generation of negative chronotropic responses to muscarinic or adenosine receptor agonists do not require activation of IK+Ach or the expression of αi2 or αi3.
Muscarinic receptor agonists activate the cardiac muscarinic K+ channel, IK+Ach, via pertussis toxin (PT)-sensitive heterotrimeric G proteins (the Gi/Go family; see refs. 1 and 2). Although cardiac muscarinic receptors can activate several G proteins with different PT-sensitive α subunits in intact cells, we do not know which G proteins in heart (Gi2, Gi3, or Go) are actually involved in signaling to the K+ channel (1–3). Reconstitution studies have clearly demonstrated that the βγ complex is directly responsible for mediating channel activation (2, 4, 5), but in these studies nearly all combinations of βγ tested (except β1γ1) were equally effective in stimulating IK+Ach. In intact cardiocytes, it appears that only those βγ complexes released from PT-sensitive heterotrimers activate the channel (1, 2). These observations have led to the suggestion that it is the α subunit of the G proteins associated with the muscarinic receptors that provides specificity to the signaling process (5).
To determine which of the PT-sensitive G proteins are involved in IK+Ach regulation in cardiocytes, we have generated a series of mutant embryonic stem cell (ES cell) lines with targeted inactivations of specific G protein α subunits. The ES cells undergo homologous recombination at high frequency, making targeted gene inactivations practical (6). In contrast to other means of achieving gene or protein inactivation (e.g., antisense expression or antibody injections), gene targeting by homologous recombination allows clear confirmation of the specificity and completeness of the gene inactivation. It also allows analysis of responses in a genetically homogeneous population of cells and generates a stable resource. Equally important, by altering culture conditions, ES cells will differentiate into a number of different cell types, including spontaneously contracting cardiac-like cells (6–11); this enables us to examine the effects of different α subunit inactivations in the specific cell type of interest.
The ES cell-derived cardiocytes have proven a valuable model in studies of the developmental regulation of cardiac gene expression (7), in analysis of chronotropic responses to cardiac receptor agonists (8), and in studies of cell cycle withdrawal by cardiocytes (9). These cardiocytes have also been shown capable of repopulating myocardium after injury (12). Electrophysiologic characterizations of the ES cell-derived cardiocytes have confirmed expression of cardiac ion channels, including the cardiac muscarinic K+ channel, IK+Ach (10, 11). This is also the first cardiocyte model that readily lends itself to genetic manipulation.
Our results show that inactivation of the genes encoding αi2 or αi3 in ES cell-derived cardiocytes blocked muscarinic receptor activation of IK+Ach but had no significant effect on negative chronotropic responses. Regulation of IK+Ach appears to specifically require expression of these αi subunits because inactivation of αo or the PT-insensitive protein αq had no effect on receptor-mediated channel activation. Identical results were observed for the cardiac adenosine receptor that also regulates IK+Ach and negative chronotropy through PT-sensitive signaling pathways. These findings demonstrate that two types of heterotrimeric G proteins, Gi2 and Gi3, are required for receptor-mediated activation of IK+Ach in intact ES cell-derived cardiocytes. Further, our data show that generation of negative chronotropic responses to muscarinic and adenosine receptors can occur in the absence of αi2 and αi3 and without activation of IK+Ach.
METHODS
Cell Culture.
Undifferentiated D3 ES cells were routinely cultured and differentiated as described (11, 13, 14). After 7 to 10 days of differentiation, single contracting cardiocytes were prepared by collagenase digestion or clusters of contracting cardiocytes mechanically separated from the surrounding cells (10, 11). Experiments were performed only with spontaneously contracting cardiac-like cells.
Gene Targeting and Isolation of Null Cell Lines.
Inactivations of the genes encoding αi2 and αi3 were performed using a single targeting construct approach as described (14, 15). The targeting constructs for inactivation of αo and αq were engineered using genomic clones isolated from a 129SV/J library. Successful gene targeting was confirmed by Southern blot analysis of clones surviving the two-step selection protocol.
Analysis of α Subunit Transcripts in Mutant Cell Lines.
For analysis of αo expression, total RNA was prepared from 7- to 10-day-old differentiated cell populations and was analyzed by Northern blot (20 μg of RNA per lane; see refs. 13 and 14). Reverse transcription (RT)–PCR was performed using the following primer pairs to discriminate between αq and the closely related protein α11: αq, 5′-GACCCTTCCTATCTGCCTACACAAC-3′ and 5′-GTTTTCCGCAGAAATACAGTCCC-3′; α11, 5′-TCATCTTCAGGATGGTGGATGTG-3′ and 5′-TGAGGAAGAAGGGACAGGACAGGA-3′. RT-PCR was performed using a Perkin–Elmer RNA–DNA kit according to the manufacturer’s instructions except that RT was carried out at 37°C for 15 min using 1 μg of total RNA prepared from undifferentiated ES cells followed by PCR amplification for 35 cycles of 95°C for 15 sec, 65°C for 30 sec, and 72°C for 15 min.
Western Blot Analysis.
Membranes were prepared as described (13) except that 0.1 mM of phenylmethylsulfonylfluoride, 1.0 mM of ethylene-diamine-tetra-acetic acid, 10 μM of leupeptin, 1.0 mM of benzamidine, and 0.1 μg/ml of aprotinin were added to the homogenization buffer. After separation (20 μg of protein per lane) on 10% SDS/PAGE, proteins were transferred to nitrocellulose, incubated with specific antibodies [anti-αi3/α0 1:1000, Calbiochem; anti-αi1/αi2 (AS/7) 1:1000, NEN; and anti-βcommon 1:1000, Upstate Biotechnology, Lake Placid, NY] and detected with the Pierce chemiluminescence system according to manufacturer’s directions.
Restoration of αi Expression.
Null cells were transfected by electroporation with 25 μg of an αi2 or αi3 cDNA expression plasmid containing a colinear hygromycin or puromycin resistance gene, respectively, as the selectable marker. All genes were expressed from a phosphoglycerate kinase promoter (13, 14). Stable transfectants were isolated in 0.2 mg/ml of hygromycin or 2.0 μg/ml of puromycin, clonally expanded; cDNA expression was confirmed by Western blot analysis.
Electrophysiological Measurements.
Whole cell K+ currents were recorded at ambient temperature using standard voltage-clamp techniques (16). The recording pipette contained 90 mM aspartate, 10 mM EGTA, 10 mM Hepes, 3 mM Na-ATP, 0.1 mM GTP, and 6 mM MgCl2 (pH 7.3). The bath solution contained 140 mM NaCl, 5.4 mM KCl, 5 mM Hepes, 10 mM glucose, 1.8 mM CaCl2, and 1.0 mM MgCl2 (pH 7.4). Tetrodatoxin (30 μM) and nifedipine (5 μM) were added to the bath solution to block the fast INa+ and ICa-L, respectively. Currents were measured by the ramp voltage clamp method with voltage applied from −150 to +70 mV at a rate of 100 mV per sec.
Single channel recordings were performed in a cell-attached configuration (16) at 21–23°C using pipettes with a tip resistance of 8–10 MΩ and seal resistance of >10 GΩ. Currents were measured with an integrating patch-clamp amplifier, filtered at 3 kHz, and analyzed with pclamp software. Bath solutions contained 145 mM NaCl, 4.0 mM KCl, 0.5 mM CaCl2, 0.5 mM MgCl2, 5.0 mM glucose, and 5.0 mM Hepes (pH 7.4). Pipette solutions contained 150 mM KCl, 1.0 mM CaCl2, 0.5 mM MgCl2, and 5.0 mM Hepes (pH 7.4). After stable basal recordings were obtained, the recording pipette was perfused with agonist-containing (10 μM carbachol or adenosine) solution. In a few experiments K+ channel activity was monitored in separate recordings either without (basal) or with agonist in the pipette. Results obtained with this configuration were identical to those obtained by pipette perfusion and the data from the two experimental protocols were pooled.
To assess IK+Ach activation by guanosine 5′-[γ-thio]triphosphate (GTP[γS]), single channel recordings were performed in an excised inside-out patch configuration (17).
Spontaneous Contraction Rates.
Contraction rates were monitored at 37°C as Ca2+ transients after loading spontaneously contracting clusters of cells with the fluorescent indicator (5 μM) fura 2-AM (18). After a stable basal recording was obtained, carbachol or adenosine (10 μM) was applied and the rate was monitored both visually and as transient changes in fluorescence intensity. Basal rates of contraction were compared with rates of contraction at peak inhibition after addition of agonists and results expressed as percent of the basal rate (agonist treated at peak inhibition/basal rate × 100%).
RESULTS
Isolation and Characterization of Mutant Cell Lines.
Mouse D3 ES cells lacking expression of one of the PT-sensitive α subunits αi2, αi3, αo, or the PT-insensitive protein αq were generated by gene targeting with a single targeting construct as described in Fig. 1. Previously, αi-null cell lines were isolated using the CCE and CC1.2 ES cell lines (13, 14, 19). We retargeted these genes and targeted the αo and αq genes in a single background, the D3 ES cell line, that reproducibly undergoes in vitro differentiation.
Figure 1.
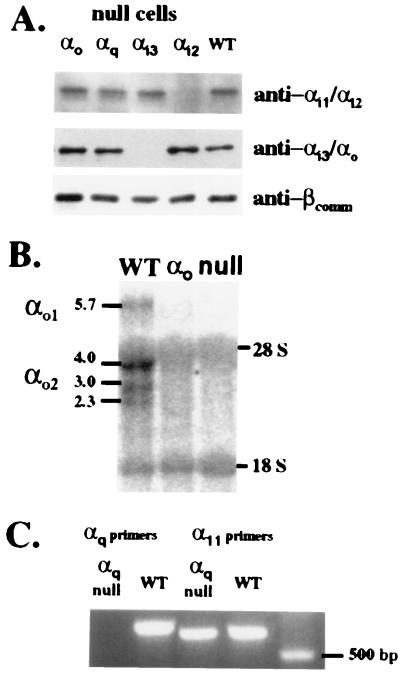
Targeting constructs for inactivation of αi2, αi3, αo, and αq and results of screening by Southern blot analysis. (A) Exon 6 of αi2 was interrupted with a neomycin (neo) resistance cassette. The predicted change in the BamHI (B) restriction digest pattern (increase in size from 4.0 to 8.0 kb) when probed with the indicated gene fragment (shaded bar) is shown below the gene maps. (B Right) The confirmation on Southern blot. For αi3, exon 1 was interrupted with a neo cassette and the ATG initiation codon for translation deleted. Southern blot analysis demonstrated the predicted increase in fragment size (from 5.2 to 6.2 kb) with inactivation of the gene. (C) The targeting construct for inactivation of αo interrupted exon 3 with a neo cassette resulting in a 1.2-kb increase in the BamHI fragment size after homologous recombination. This targeting construct was designed such that both splice variants of αo would be eliminated. (D) For αq, exon 6 was interrupted with a neo cassette resulting in a 1.5-kb increase in the XbaI (X) fragment on Southern blot. Endog, endogenous gene; HR, gene after homologous recombination; SKO, single knockout, heterozygous mutant clone.
After screening clones by Southern blot analysis for disruption of both alleles (Fig. 1), successful inactivation of the targeted gene was confirmed (Fig. 2) by evaluating the cells for protein (αi2 and αi3) or for transcript (αo and αq). Targeting of the genes for specific α subunits had no effects on expression of nontargeted αi2, αi3, or β subunits in ES cells (Fig. 2A). There appeared to be little αi1 and no detectable αo in the undifferentiated cells. We also noted no compensatory changes in the amounts of the other G protein subunits detected on the blots.
Figure 2.
Confirmation of α subunit gene inactivations in the mutant ES cell lines. (A) Western blot analysis of membrane protein from undifferentiated WT and α-null cells. (B) Northern blot analysis of total RNA prepared from 7- to 10-day-old differentiated cells and probed with a 1.1-kb NcoI–EcoRI fragment of αo cDNA containing exons 3 through 8 revealed the presence of multiple transcripts in WT cells but none in the αo-null cells. Non-specific binding to 18S and 28S RNA was observed in all lanes. (C) RT-PCR of total RNA was used to assess αq expression and expression of the highly related α11 gene product. An αq product of the predicted size of 655 bp was amplified only in WT cells while the control amplification for α11 detected a product (predicted size of 589 bp) in both WT and the αq-null cells.
Wild-type (WT) and mutant ES cells were then allowed to differentiate in culture and spontaneously contracting cardiocytes isolated (11). All cell lines produce spontaneously contracting cardiocytes by day 10 or 11, and the proportion and morphologic appearance of the contracting cells was indistinguishable between WT and mutant cell lines.
Characterization of IK+Ach in ES Cell-Derived Cardiocytes.
Analysis of whole cell currents in cardiocytes derived from WT ES cells (Fig. 3A) showed the characteristic stimulation of the IK+ after perfusion of the chamber with the muscarinic agonist carbachol (IK+ at −140 mV: 0.23 ± 0.01 nA basal vs. 0.74 ± 0.05 nA carbachol, n = 5, P < 0.001). This stimulation was completely blocked by pretreatment of the cells with the m2 muscarinic receptor antagonist, methoctramine (Fig. 3B). In ES cell-derived cardiocytes lacking expression of αi2 or αi3 (Fig. 3 C and D), carbachol did not substantially increase the whole cell K+ currents (IK+ at −140 mV: αi2-null 0.23 ± 0.01 nA basal vs. 0.30 ± 0.05 nA carbachol, n = 4; NS and αi3-null 0.24 ± 0.02 nA basal vs. 0.31 ± 0.03 nA carbachol, n = 3, NS).
Figure 3.
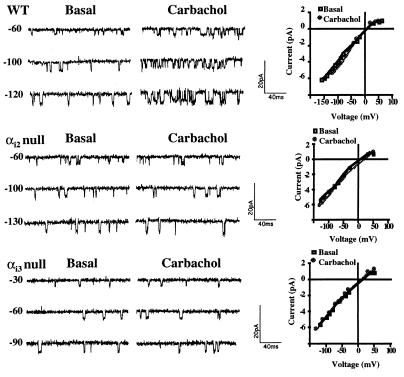
Whole cell K+ currents from WT and αi-null ES cell-derived cardiocytes are illustrated. (A) With WT cells a marked increase from basal (a) current was seen after perfusion of the recording chamber with (b) 10 μM carbachol. (B) Addition of the m2 muscarinic receptor antagonist (b) methoctramine (50 nM) did not affect basal current (a) but blocked the subsequent stimulation by carbachol (c). With αi2-null cells (C) and αi3-null cells (D) basal currents (a) were similar to WT cells but did not substantially increase after addition of carbachol (b).
We further characterized the single K+ channel activities in the ES cell-derived cardiocytes using on-cell attached patches. With this approach we verified expression of an inwardly rectifying K+ channel consistent with IK+Ach (2, 11). A typical current trace for WT cells is shown in Fig. 4 and demonstrates low basal channel activity and a marked increase in channel openings after perfusion of the patch pipette with the muscarinic receptor agonist, carbachol. The current–voltage (I–V) relationship for this channel demonstrates inward rectification at positive voltages and a slope conductance of 46 ± 0.6 pS (n = 20) at 150 mM of extracellular K+. The absence of a carbachol effect on channel conductance (43 ± 1.0 pS, n = 10, with carbachol) or mean open time (0.58 ± 0.05 msec, n = 10, basal vs. 0.52 ± 0.04 msec, n = 8, with carbachol) is characteristic for IK+Ach and indicates that the increased channel activity noted in the presence of agonist results from an increase in channel open state probability.
Figure 4.
Single channel recordings of the inwardly rectifying K+ channel, IK+Ach, in WT, αi2-null, and αi3-null ES cell-derived cardiocytes. After stable basal recordings were obtained and single channel activities at different voltages recorded, the pipette was perfused with 10 μM of carbachol. Holding voltages (in mV) are shown to the left of the current traces. The I–V relationships for IK+Ach recorded in WT, αi2-null, and αi3-null cells at 150 mM KCl in the absence (basal) and presence of carbachol (10 μM) are similar as shown to the right of the single channel current traces.
Identical electrophysiological parameters were obtained for the K+ channels recorded from cardiocytes isolated from the α subunit-null cells, thus confirming the presence of IK+Ach in the mutant cell lines. Current traces and I–V curves for αi2- and αi3-null cells are shown in Fig. 4. As with cardiocytes derived from WT cells, single channel analysis of the αi-null cardiocytes demonstrated that carbachol had no effect on inward rectification, conductance [αi2-null: 47 ± 1.6 pS basal (n = 6) vs. 48 ± 1.3 pS (n = 5) carbachol; αi3-null: 44 ± 1.9 pS basal (n = 5) vs. 42 ± 1.2 pS (n = 4) carbachol] or mean open time [αi2-null: 0.45 ± 0.08 msec basal (n = 5) vs. 0.51 ± 0.13 msec (n = 3) carbachol; αi3-null: 0.46 ± 0.08 msec basal (n = 4) vs. 0.45 ± 0.10 msec (n = 4) carbachol]. The conductance, mean open time, and inward rectification were similar for channels recorded from αo- and αq-null cells (data not shown). Resting membrane potentials of cardiocytes derived from WT and αi-null cells were in the same range (from −65 to −75 mV).
Effect of α Subunit Gene Inactivations on Regulation of IK+Ach Activity by Carbachol.
Comparison of K+ channel activity (expressed as NPo, where N is the number of current levels observed and Po is the single channel open state probability) among the cell lines revealed no significant differences in basal NPo (Fig. 5A). With addition of carbachol, channel activity increased approximately fourfold in the WT and was unaffected by targeted disruption of the PT-sensitive subunit αo or the PT-insensitive subunit αq (Fig. 5A). Pretreatment of WT, αo-null, and αq-null cardiocytes with PT (0.1 μg/ml for 18 h) completely blocked stimulation of IK+Ach activity by carbachol (data not shown). Both the basal- and agonist-stimulated channel activity in the ES cell-derived cardiocytes were in agreement with results reported for IK+Ach activity in on-cell recordings of atrial cardiocytes from a variety of species (20–23).
Figure 5.
Effect of α subunit gene inactivations on receptor-mediated IK+Ach activation. (A) Comparison of IK+Ach activity expressed as NPo before (basal) and after addition of carbachol (10 μM) in WT, α-null cells, and αi-R cells in which the targeted gene has been reexpressed. Bars are means ± SE for 10–20 recordings for WT and αi-null cells and 4 to 5 recordings for αi-R, αo-null, and αq-null cells. (B) Western blot of membrane protein from undifferentiated WT, αi-null cells, and αi-R cells in which the targeted gene (αi2 or αi3) has been reexpressed demonstrate the absence of protein in the null cells but significant protein in the rescued (αi-R) cell lines.
Rescue of αi-Null Cell Lines by Expression of the cDNA.
With cardiocytes derived from ES cells lacking αi2 or αi3, we consistently found that carbachol did not increase channel activity (Fig. 5A). To test whether the observed phenotypes in the αi-null cells resulted from an unrecognized effect of the selection process or an incidentally acquired mutation, we examined the effect of restoring αi expression in null cells on carbachol-induced channel activation. After stably reexpressing αi2 in αi2-null cells (αi2-R) and αi3 in αi3-null cells (αi3-R) (Fig. 5B), the rescued clones were differentiated and IK+Ach regulation was examined (Fig. 5A). For both αi2- and in αi3-null cells, reexpression of the previously targeted genes resulted in a marked increase in carbachol-induced channel activation (αi2-R NPo 0.15 ± 0.02 vs. αi2-null NPo 0.04 ± 0.01; αi3-R NPo 0.17 ± 0.02 vs. αi3-null NPo 0.06 ± 0.01). These results demonstrate that the failure of carbachol to activate IK+Ach in the αi-null cells is due to the absence of the targeted genes.
Regulation of IK+Ach by Cardiac Adenosine Receptors.
Like the muscarinic receptors, cardiac adenosine receptors that activate IK+Ach also signal via PT-sensitive G proteins (2). Expression of both receptor types in the same cell population allowed us to compare the signaling requirements for IK+Ach activation by these two receptor types. Interestingly, we found an identical pattern of responses to adenosine in the null cell lines. Adenosine-induced channel activation was unaffected by targeting of αo, but was completely blocked by inactivation of αi2 or of αi3 (Fig. 6).
Figure 6.
Adenosine’s effect on IK+Ach activation in WT and α-null cell lines. IK+Ach activity was measured before (basal) and after perfusion of the recording pipette with 10 μM adenosine. Bars are means ± SE for 5–8 recordings.
Agonist-Independent Regulation of IK+Ach in Cardiocytes Derived from αi-Null ES Cells.
Addition of GTP[γS] to the cytoplasmic face of excised inside-out patches from native cardiocytes results in agonist-independent activation of IK+Ach, presumably by nonspecifically activating G proteins and freeing βγ complexes (2). Since reconstitution experiments have failed to demonstrate specificity in channel activation by different βγ complexes, we reasoned that GTP[γS] should be able to activate IK+Ach in the null cell lines if specific βγ complexes from Gi2 and Gi3 were not required and if the channel itself was present and unaltered by the targeting process.
As noted by others (2, 4, 24), we found that the basal channel activity (NPo) for IK+Ach measured in an excised-patch configuration was lower than that measured in the cell-attached patches (Fig. 7 compared with Fig. 5A). This most likely results from differences in the composition of cellular cytoplasm and the solution used in the excised patch mode. With addition of 100 μM of GTP[γS] to the bath solution, we observed an identical increase in IK+Ach activity in the WT and αi-null cells, clearly demonstrating that the channels are present in an activatable state (Fig. 7).
Figure 7.
Agonist-independent activation of IK+Ach by 100 μM of GTP[γS] in inside-out patches excised from WT, αi2-null, and αi3-null cells. Shown are typical current traces recorded before (basal) and after addition of GTP[γS] to the bath solution. Activation of IK+ATP was blocked by addition of Mg-ATP (200 μM) to the bath solution. Quantitative estimates of channel activity expressed as NPo are shown in the bottom panel. Bars are the mean ± SE (n = 5–6) before (basal) and after addition of GTP[γS].
The number of current levels observed with this mode of single channel recording revealed that the density of IK+Ach channels in membrane patches from αi-null cells was nearly identical to that of the WT ES cell-derived cardiocytes (current levels/patch: WT, 2.0 ± 0.3; αi2-null, 2.0 ± 0; αi3-null, 2.0 ± 0.3, n = 5 separate patches for each cell type: ≈1.27 channels per μm2 membrane area). Depending upon the species studied and the method used to determine channel density in isolated atrial patches, values ranging from 0.5 to 9.0 channels per μm2 have been reported (20, 23, 25).
Effect of αi-Null Mutations on Negative Chronotropic Responses.
Slowing of cardiac contraction rate is a well recognized effect of muscarinic and adenosine receptor agonists (26); ES cell-derived cardiocytes exhibit typical chronotropic responses to a number of agonists, including those for muscarinic receptors (8). We found that the negative chronotropic response to carbachol was blocked in WT cells by pretreatment with an m2 receptor antagonist, methoctramine (50 nM), consistent with the responses being generated by the m2 receptor subtype (data not shown). We also confirmed that the chronotropic response to carbachol in ES cell-derived cardiocytes, like that of native heart cells, is blocked by PT (Fig. 8).
Figure 8.
The effects of carbachol or adenosine (both 10 μM) on the spontaneous contraction rate of the ES cell-derived cardiocytes were examined in isolated clusters of contracting cells. The effect of pretreatment of cells with PT (0.1 μg/ml for 18 h) on carbachol-induced slowing is also shown. Results are expressed as percent (%) of basal rate calculated as (agonist treated/basal × 100%). Bars are means ± SE for 9–25 paired measurements.
In isolated clusters of contracting ES cell-derived cardiocytes, the basal rate of spontaneous contraction was 65 ± 5 bpm (n = 20) for WT cells and was not affected by inactivation of the genes for αi2 (basal rate 66 ± 7 bpm, n = 19) or αi3 (basal rate 58 ± 5 bpm, n = 14). Unlike IK+Ach regulation, both muscarinic and adenosine receptor-mediated negative chronotropic responses were unaffected by the absence of αi2 or αi3 in the ES cell-derived cardiocytes. A 40–60% inhibition of the spontaneous contraction rate was observed in WT and mutant cell lines and with both agonists (Fig. 8). Targeting of αi2 and αi3 also did not alter the PT-sensitivity of the negative chronotropic response to carbachol. Preservation of the negative chronotropic responses confirmed that functional receptors are present in the null cells and that PT-sensitive G proteins are still involved in the signaling process.
DISCUSSION
Using ES cell-derived cardiocytes with targeted inactivations of specific G protein α subunits, we have shown that expression of both αi2 and αi3 is required for muscarinic and adenosine receptor-mediated regulation of IK+Ach in intact cells. Previous studies of muscarinic receptor-G protein signaling mechanisms have reported that either a single α subtype is required for signaling as in the GH3 cells (27) or, more often, that there is a preferred α subtype with other G proteins being able to substitute with varying efficacy (28–30). The requirement for the heterotrimers containing both αi2 and αi3 appears to be characteristic of this signaling pathway to the muscarinic K+ channel because we observed an identical αi subunit requirement for activation of IK+Ach by both muscarinic and adenosine receptors.
Recent data indicate that regulation of IK+Ach activity is more complex than previously appreciated. The channel is a large heteromultimer composed of at least two different proteins, GIRK 1 (Kir 3.1) and CIR (GIRK 4 or Kir 3.4), both of which possess βγ binding sites (4, 31, 32). The stoichiometry of βγ binding required for channel activation in intact cardiocytes is not known. Recent studies with cloned subunits of the channel expressed in Xenopus suggest that binding of βγ complexes at multiple sites on both GIRK1 and CIR are required for maximal activation of IK+Ach (32). Interestingly, several observations suggest that the muscarinic K+ channel may also directly interact with G protein α subunits to enhance the α subunit GTPase activity (for review, see ref. 2). Furthermore, a recent report demonstrated that activated (GTP-bound) αs and αi1 (and perhaps αo) can inhibit βγ-mediated activation of IK+Ach in excised inside-out patches (33). The significance of the αi1 effect is unclear as this PT-sensitive subtype is apparently not expressed in heart (34).
It seems unlikely that functional differences between the βγ complexes contributed by the heterotrimers Gi2 and Gi3 account for the dual G protein requirement given that IK+Ach activation in reconstitution studies exhibits little βγ subtype specificity (2, 4, 5). Also, our data show that there is not an absolute requirement for the specific βγ complexes released from Gi2 and Gi3 because GTP[γS] can fully activate IK+Ach in the αi-null cells. It is possible that receptor activation of both Gi2 and Gi3 is needed to release sufficient numbers of βγ complexes to activate the channel. Importantly, inactivation of α subunits in the ES cell-derived cardiocytes did not lead to unregulated activation of IK+Ach. This differs from the yeast system where inactivation of the α subunit leads to constitutive activation of βγ regulated effectors (35). Our studies also do not rule out a direct or indirect role for αi2 or αi3 in modulating channel activity.
Failure of IK+Ach to respond to agonist is not due to an absence of the channel in the αi-null cells since it is activated by GTP[γS]. Nor does there appear to be an absence of receptors given the retention of negative chronotropic responses to carbachol and adenosine. We also have evidence (data not shown) that cells lacking both αi2 and αi3 modulate whole cell Ca2+ currents in response to carbachol further supporting the presence of muscarinic receptors in the null cell lines.
Results of our chronotropy studies show that for ES cell-derived cardiocytes expression of αi2 and αi3 is not required for the slowing of contractions in response to carbachol or adenosine. The negative chronotropic response is still sensitive to PT in the mutants suggesting that other PT-sensitive G proteins (i.e., Go) are more important or can substitute in this signaling process. These studies do not rule out a role for IK+Ach in generation of the normal negative chronotropic response. Our results are consistent with data indicating that other currents such as the hyperpolarization activated current, If, are important contributors to the cardiac “pacemaker” current (26). Interestingly, in reconstitution studies using cardiac nodal cells, αo is reported to be a more potent modulator of If than are the αi subunits (36). Regulation of contraction rate is clearly a complex process and will require more detailed studies to precisely define G protein signaling requirements for the relevant effectors.
We show that muscarinic receptor-induced activation of IK+Ach in intact cells requires expression of two specific PT-sensitive G proteins, αi2 and αi3. The simplest explanation for this observation is that both Gi2 and Gi3 are involved in signal transduction to IK+Ach and that in their absence the other G proteins are not able to substitute. Additional studies with intact cardiocytes are needed to determine whether both heterotrimeric Gi2 and Gi3 do in fact contribute βγ complexes that activate IK+Ach and whether there are additional regulatory effects transmitted by specific α subunits. These studies also illustrate the usefulness of ES cell-derived in vitro differentiated cells for signaling studies.
Acknowledgments
This work is supported by grants from the National Institutes of Health (GM49122) and the American Heart Association. M.O.S. is the recipient of a Clinical Investigator Development Award. R.M.M. is an Established Investigator of the American Heart Association.
ABBREVIATIONS
- IK+Ach
cardiac muscarinic K+ channel
- PT
pertussis toxin
- ES cell
embryonic stem cell
- GTP[γS]
guanosine 5′-[γ-thio]triphosphate
- WT
wild type
- I–V
current–voltage
References
- 1.Clapham D E. Annu Rev Neurosci. 1994;17:441–464. doi: 10.1146/annurev.ne.17.030194.002301. [DOI] [PubMed] [Google Scholar]
- 2.Kurachi Y. Am J Physiol. 1995;269:C821–C830. doi: 10.1152/ajpcell.1995.269.4.C821. [DOI] [PubMed] [Google Scholar]
- 3.Migeon J C, Thomas S L, Nathanson N M. J Biol Chem. 1995;270:16070–16074. doi: 10.1074/jbc.270.27.16070. [DOI] [PubMed] [Google Scholar]
- 4.Krapivinsky G, Gordon E A, Wickman K, Velimirovic B, Krapivinsky L, Clapham D E. Nature (London) 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- 5.Wickman K D, Iniguez-Lluhl J A, Davenport P A, Taussig R, Krapivinsky G B, Linder M E, Gilman A G, Clapham D E. Nature (London) 1994;368:255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- 6.Bronson S K, Smithies O. J Biol Chem. 1994;269:27155–27158. [PubMed] [Google Scholar]
- 7.Miller-Hance W C, LaCorbiere M, Fuller S J, Evans S M, Lyons G, Schmidt C, Robbins J, Chien K R. J Biol Chem. 1993;268:25244–25252. [PubMed] [Google Scholar]
- 8.Wallukat G, Wobus A M. Arch Toxicol Suppl. 1991;14:136–139. doi: 10.1007/978-3-642-74936-0_27. [DOI] [PubMed] [Google Scholar]
- 9.Klug M G, Soonpaa M H, Field L J. Am J Physiol. 1995;269:H1913–H1921. doi: 10.1152/ajpheart.1995.269.6.H1913. [DOI] [PubMed] [Google Scholar]
- 10.Maltsev V A, Rohwedel J, Hescheler J, Wobus A M. Mech Dev. 1993;44:41–50. doi: 10.1016/0925-4773(93)90015-p. [DOI] [PubMed] [Google Scholar]
- 11.Maltsev V A, Wobus A M, Rohwedel J, Bader M, Hescheler J. Circ Res. 1994;75:233–244. doi: 10.1161/01.res.75.2.233. [DOI] [PubMed] [Google Scholar]
- 12.Klug M G, Soonpaa M H, Koh G, Y, Field L J. J Clin Invest. 1996;98:216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mortensen R M, Zubiaur M, Neer E J, Seidman J G. Proc Natl Acad Sci USA. 1991;88:7036–7040. doi: 10.1073/pnas.88.16.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mortensen R M, Conner D A, Chao S, Geisterfer-Lowrance A A T, Seidman J G. Mol Cell Biol. 1992;12:2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current Protocols in Molecular Biology. New York: Wiley; 1991. [Google Scholar]
- 16.Hammill D P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 17.Ito H, Tung R T, Sugimoto T, Kobayashi I, Takahashi K, Katada T, Ui M, Kurachi Y. J Gen Physiol. 1992;99:961–983. doi: 10.1085/jgp.99.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinn S J, Williams G H, Tillotson D L. Proc Natl Acad Sci USA. 1988;85:5754–5758. doi: 10.1073/pnas.85.15.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raymond J R, Arthur J M, Casanas S J, Olsen C L, Gettys T W, Mortensen R M. J Biol Chem. 1994;269:13073–13075. [PubMed] [Google Scholar]
- 20.Soejima M, Noma A. Pflügers Arch. 1984;400:424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- 21.Ito H, Ono K, Noma A. J Physiol (London) 1994;476:55–68. [PMC free article] [PubMed] [Google Scholar]
- 22.Koumi S, Arentzen C E, Backer C L, Wasserstrom J A. Circulation. 1994;90:2213–2224. doi: 10.1161/01.cir.90.5.2213. [DOI] [PubMed] [Google Scholar]
- 23.Koumi S-I, Wasserstrom J A. Am J Physiol. 1994;266:H1812–H1821. doi: 10.1152/ajpheart.1994.266.5.H1812. [DOI] [PubMed] [Google Scholar]
- 24.Kurachi Y, Nakajima T, Sugimoto T. Pflügers Arch. 1986;407:264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- 25.Ito H, Hosoya Y, Inanobe A, Tomoike H, Endoh M. Naunyn-Schmiedeberg’s Arch Pharmacol. 1995;351:610–617. doi: 10.1007/BF00170160. [DOI] [PubMed] [Google Scholar]
- 26.DiFrancesco D. Annu Rev Physiol. 1993;55:455–472. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- 27.Kleuss C, Hescheler J, Ewel C, Rosenthal W, Schultz G, Wittig B. Nature (London) 1991;353:43–48. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- 28.Hunt T W, Carroll R C, Peralta E G. J Biol Chem. 1994;269:29565–29570. [PubMed] [Google Scholar]
- 29.Migeon J C, Thomas S L, Nathanson N M. J Biol Chem. 1994;269:29146–29152. [PubMed] [Google Scholar]
- 30.Rudolph U, Spicher K, Birnbaumer L. Proc Natl Acad Sci USA. 1996;93:3209–3214. doi: 10.1073/pnas.93.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krapivinsky G, Krapivinsky L, Wickman K, Clapham D E. J Biol Chem. 1995;270:29059–29062. doi: 10.1074/jbc.270.49.29059. [DOI] [PubMed] [Google Scholar]
- 32.Tucker S J, Pessia M, Adelman J P. Am J Physiol. 1996;271:H379–H385. doi: 10.1152/ajpheart.1996.271.1.H379. [DOI] [PubMed] [Google Scholar]
- 33.Schreibmayer W, Dessauer C W, Vorobiov D, Gilman A G, Lester H A, Davidson N, Dascal N. Nature (London) 1996;380:624–627. doi: 10.1038/380624a0. [DOI] [PubMed] [Google Scholar]
- 34.Luetje C W, Tietje K M, Christian J L, Nathanson N M. J Biol Chem. 1988;263:13357–13365. [PubMed] [Google Scholar]
- 35.Jahng K Y, Fergusin J, Reed S I. Mol Cell Biol. 1988;8:2484–2493. doi: 10.1128/mcb.8.6.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yatani A, Okabe K, Codina J, Birnbaumer L, Brown A M. Science. 1990;249:1163–1166. doi: 10.1126/science.1697697. [DOI] [PubMed] [Google Scholar]