Abstract
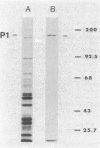
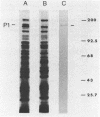
A 165,000-dalton surface protein of Mycoplasma pneumoniae, designated protein P1, appears to be the major attachment ligand of the pathogen. We employed monoclonal antibody affinity chromatography to obtain purified protein P1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson K. K., Benyamin Y., Douzou P., Balny C. The effects of organic solvents and temperature on the desorption of yeast 3-phosphoglycerate kinase from immunoadsorbent. J Immunol Methods. 1979;25(4):375–381. doi: 10.1016/0022-1759(79)90029-2. [DOI] [PubMed] [Google Scholar]
- Banai M., Razin S., Bredt W., Kahane I. Isolation of binding sites to glycophorin from Mycoplasma pneumoniae membranes. Infect Immun. 1980 Dec;30(3):628–634. doi: 10.1128/iai.30.3.628-634.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Cole R. M., Krause D. C., Leith D. K. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982 Sep;151(3):1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Feldner J., Göbel U., Bredt W. Mycoplasma pneumoniae adhesin localized to tip structure by monoclonal antibody. Nature. 1982 Aug 19;298(5876):765–767. doi: 10.1038/298765a0. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Surface parasitism by Mycoplasma pneumoniae of respiratory epithelium. J Exp Med. 1977 May 1;145(5):1328–1343. doi: 10.1084/jem.145.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Baseman J. B. Inhibition of mycoplasma pneumoniae hemadsorption and adherence to respiratory epithelium by antibodies to a membrane protein. Infect Immun. 1983 Mar;39(3):1180–1186. doi: 10.1128/iai.39.3.1180-1186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Baseman J. B. Mycoplasma pneumoniae proteins that selectively bind to host cells. Infect Immun. 1982 Jul;37(1):382–386. doi: 10.1128/iai.37.1.382-386.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leith D. K., Trevino L. B., Tully J. G., Senterfit L. B., Baseman J. B. Host discrimination of Mycoplasma pneumoniae proteinaceous immunogens. J Exp Med. 1983 Feb 1;157(2):502–514. doi: 10.1084/jem.157.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman R. P., Clyde W. A., Jr, Denny F. W. Characteristics of virulent, attenuated, and avirulent Mycoplasma pneumoniae strains. J Bacteriol. 1969 Nov;100(2):1037–1043. doi: 10.1128/jb.100.2.1037-1043.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison-Plummer J., Jones D. H., Baseman J. B. An ELISA to detect monoclonal antibodies specific for lipid determinants of Mycoplasma pneumoniae. J Immunol Methods. 1983 Nov 11;64(1-2):165–178. doi: 10.1016/0022-1759(83)90395-2. [DOI] [PubMed] [Google Scholar]