Abstract
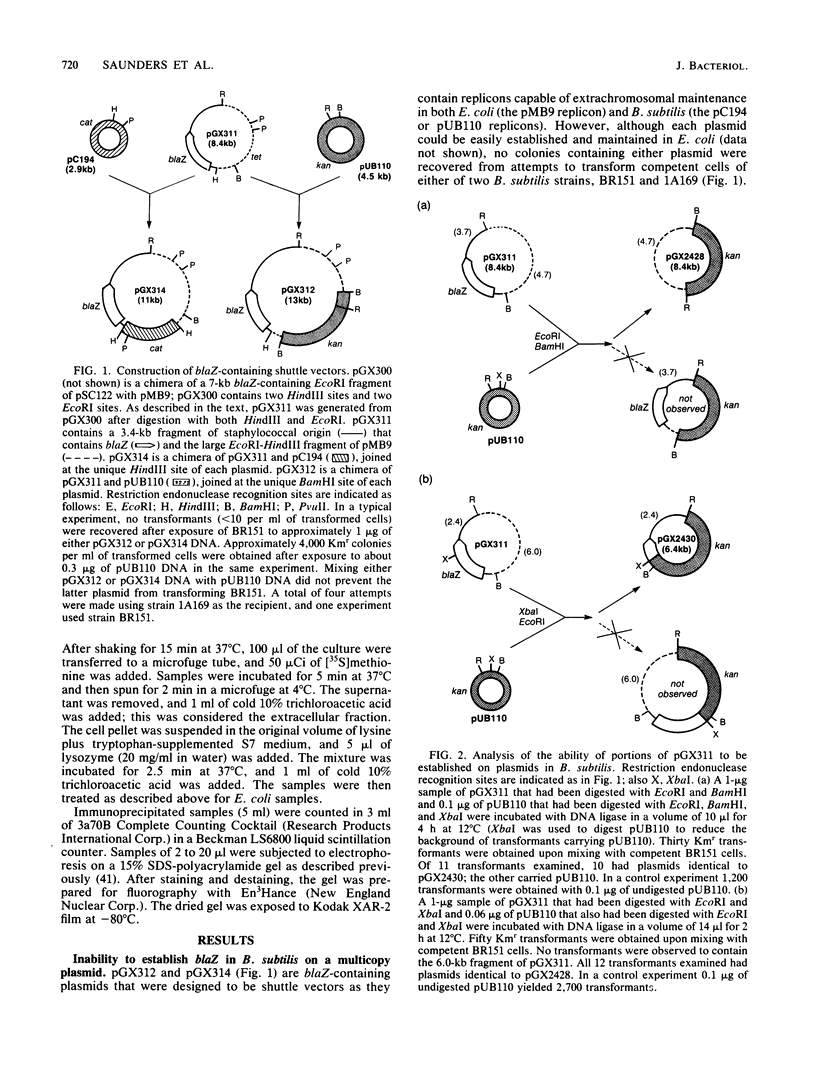
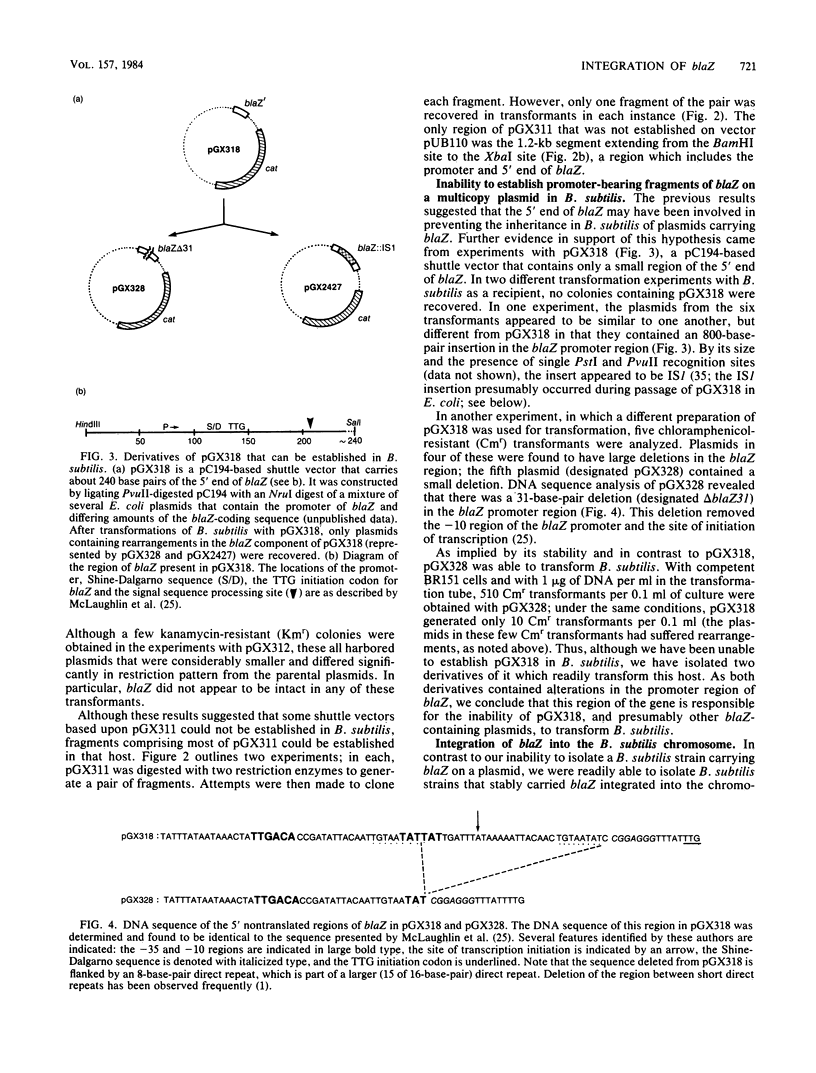
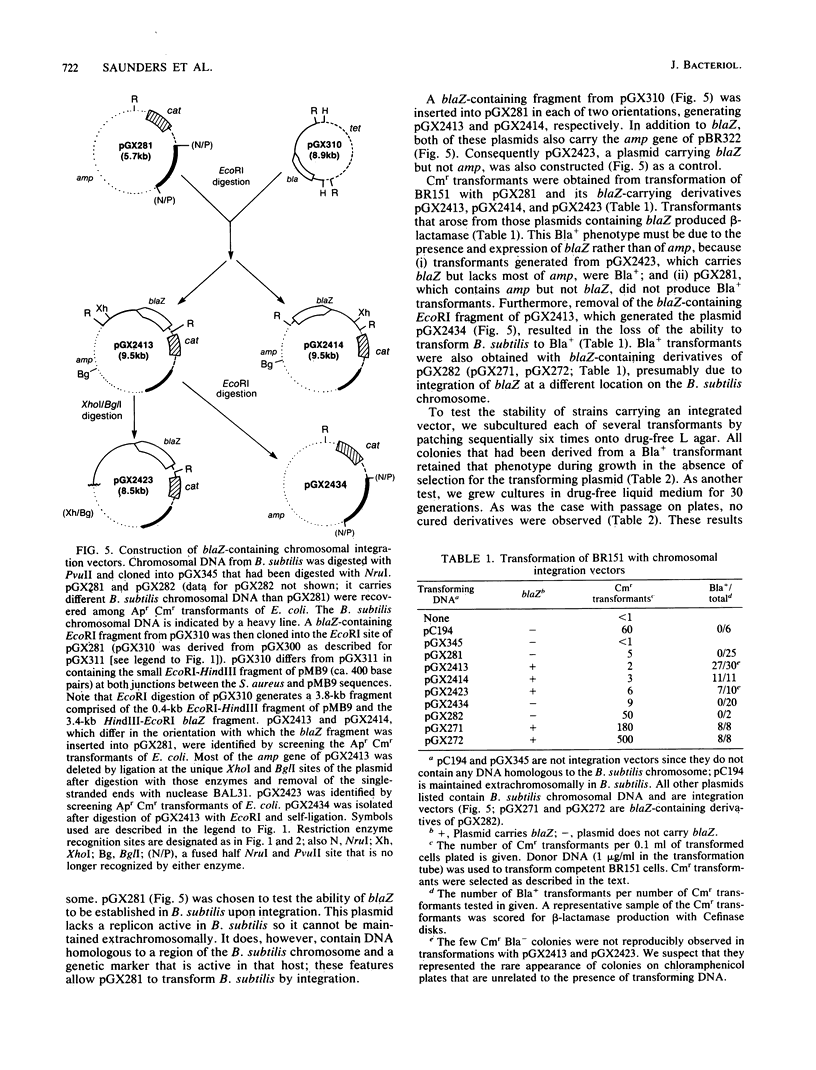
With several different vectors, attempts were made to establish blaZ, a Staphylococcus aureus beta-lactamase gene, in Bacillus subtilis. Stable establishment of blaZ in B. subtilis was achieved by use of a vector that allowed the integration of a single copy of the gene into the chromosome of that host. blaZ was expressed in the heterologous host since B. subtilis strains carrying integrated blaZ produced beta-lactamase and were more resistant to ampicillin than was wild-type B. subtilis. blaZ was stably inherited in such strains, as no loss of the ability to produce beta-lactamase was observed after growth in nonselective liquid medium or on solid medium. In contrast, a blaZ-containing restriction fragment could not be established in B. subtilis with either pUB110- or pC194-based vectors. Similarly, a pC194-based shuttle vector (pGX318) containing the 5' end of blaZ (including the promoter and the coding region for the signal sequence and the first few amino acids of the mature protein) was unable to transform B. subtilis. Two derivatives of pGX318 that could be stably established in B. subtilis were isolated. The structures of these derivatives suggested that inactivation of the blaZ promoter was associated with the acquisition of the ability to be established.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Ambler R. P. The amino acid sequence of Staphylococcus aureus penicillinase. Biochem J. 1975 Nov;151(2):197–218. doi: 10.1042/bj1510197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Ehrlich S. D., Niaudet B., Michel B. Use of plasmids from Staphylococcus aureus for cloning of DNA in Bacillus subtilis. Curr Top Microbiol Immunol. 1982;96:19–29. doi: 10.1007/978-3-642-68315-2_2. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentz R., Langner A., Chang A. C., Cohen S. N., Bujard H. Cloning and analysis of strong promoters is made possible by the downstream placement of a RNA termination signal. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4936–4940. doi: 10.1073/pnas.78.8.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray O., Chang S. Molecular cloning and expression of Bacillus licheniformis beta-lactamase gene in Escherichia coli and Bacillus subtilis. J Bacteriol. 1981 Jan;145(1):422–428. doi: 10.1128/jb.145.1.422-428.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer M. S., Clark A. J. cis-Dominant, transfer-deficient mutants of the Escherichia coli K-12 F sex factor. J Bacteriol. 1976 Jan;125(1):233–247. doi: 10.1128/jb.125.1.233-247.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer M. S. The gamma delta sequence of F is an insertion sequence. J Mol Biol. 1978 Dec 15;126(3):347–365. doi: 10.1016/0022-2836(78)90045-1. [DOI] [PubMed] [Google Scholar]
- Haldenwang W. G., Banner C. D., Ollington J. F., Losick R., Hoch J. A., O'Connor M. B., Sonenshein A. L. Mapping a cloned gene under sporulation control by inserttion of a drug resistance marker into the Bacillus subtilis chromosome. J Bacteriol. 1980 Apr;142(1):90–98. doi: 10.1128/jb.142.1.90-98.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding N. E., Cleary J. M., Smith D. W., Michon J. J., Brusilow W. S., Zyskind J. W. Chromosomal replication origins (oriC) of Enterobacter aerogenes and Klebsiella pneumoniae are functional in Escherichia coli. J Bacteriol. 1982 Dec;152(3):983–993. doi: 10.1128/jb.152.3.983-993.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U., Royer H. D., Teather R. M., Hindennach I., Hollenberg C. P. Cloning of the structural gene (ompA) for an integral outer membrane protein of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4360–4364. doi: 10.1073/pnas.76.9.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka T., Tanaka T., Tsunekawa H., Aiba S. Cloning of the genes for penicillinase, penP and penI, of Bacillus licheniformis in some vector plasmids and their expression in Escherichia coli, Bacillus subtilis, and Bacillus licheniformis. J Bacteriol. 1981 Sep;147(3):776–786. doi: 10.1128/jb.147.3.776-786.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Duvall E. J., Keggins K. M. Bacillus pumilus plasmid pPL10: properties and insertion into Bacillus subtilis 168 by transformation. J Bacteriol. 1976 Aug;127(2):817–828. doi: 10.1128/jb.127.2.817-828.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [PubMed] [Google Scholar]
- Michaelis S., Guarente L., Beckwith J. In vitro construction and characterization of phoA-lacZ gene fusions in Escherichia coli. J Bacteriol. 1983 Apr;154(1):356–365. doi: 10.1128/jb.154.1.356-365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Calos M. P., Galas D., Hofer M., Büchel D. E., Müller-Hill B. Genetic analysis of transpositions in the lac region of Escherichia coli. J Mol Biol. 1980 Nov 25;144(1):1–18. doi: 10.1016/0022-2836(80)90212-0. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Méjean V., Claverys J. P., Vasseghi H., Sicard A. M. Rapid cloning of specific DNA fragments of Streptococcus pneumoniae by vector integration into the chromosome followed by endonucleolytic excision. Gene. 1981 Nov;15(2-3):289–293. doi: 10.1016/0378-1119(81)90139-6. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaudet B., Goze A., Ehrlich S. D. Insertional mutagenesis in Bacillus subtilis: mechanism and use in gene cloning. Gene. 1982 Oct;19(3):277–284. doi: 10.1016/0378-1119(82)90017-8. [DOI] [PubMed] [Google Scholar]
- Nielsen J. B., Lampen J. O. Membrane-bound penicillinases in Gram-positive bacteria. J Biol Chem. 1982 Apr 25;257(8):4490–4495. [PubMed] [Google Scholar]
- Nisen P., Purucker M., Shapiro L. Deoxyribonucleic acid sequence homologies among bacterial insertion sequence elements and genomes of various organisms. J Bacteriol. 1979 Nov;140(2):588–596. doi: 10.1128/jb.140.2.588-596.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Murphy E., Gryczan T. J., Baron E., Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979 Jan;2(1):109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Ohtsubo H., Ohtsubo E. Nucleotide sequence of an insertion element, IS1. Proc Natl Acad Sci U S A. 1978 Feb;75(2):615–619. doi: 10.1073/pnas.75.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder G. S., Fink G. R. DNA rearrangements associated with a transposable element in yeast. Cell. 1980 Aug;21(1):239–249. doi: 10.1016/0092-8674(80)90131-2. [DOI] [PubMed] [Google Scholar]
- Sonenshein A. L., Cami B., Brevet J., Cote R. Isolation and characterization of rifampin-resistant and streptolydigin-resistant mutants of Bacillus subtilis with altered sporulation properties. J Bacteriol. 1974 Oct;120(1):253–265. doi: 10.1128/jb.120.1.253-265.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassi D. L., Lacks S. A. Effect of strong promoters on the cloning in Escherichia coli of DNA fragments from Streptococcus pneumoniae. Gene. 1982 Jun;18(3):319–328. doi: 10.1016/0378-1119(82)90170-6. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasantha N., Freese E. Enzyme changes during Bacillus subtilis sporulation caused by deprivation of guanine nucleotides. J Bacteriol. 1980 Dec;144(3):1119–1125. doi: 10.1128/jb.144.3.1119-1125.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Duvall E. J., Lovett P. S. Cloning restriction fragments that promote expression of a gene in Bacillus subtilis. J Bacteriol. 1981 Jun;146(3):1162–1165. doi: 10.1128/jb.146.3.1162-1165.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F. E., Hoch J. A., Bott K. Genetic mapping of a linked cluster of ribosomal ribonucleic acid genes in Bacillus subtilis. J Bacteriol. 1981 Nov;148(2):624–628. doi: 10.1128/jb.148.2.624-628.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]




