Abstract
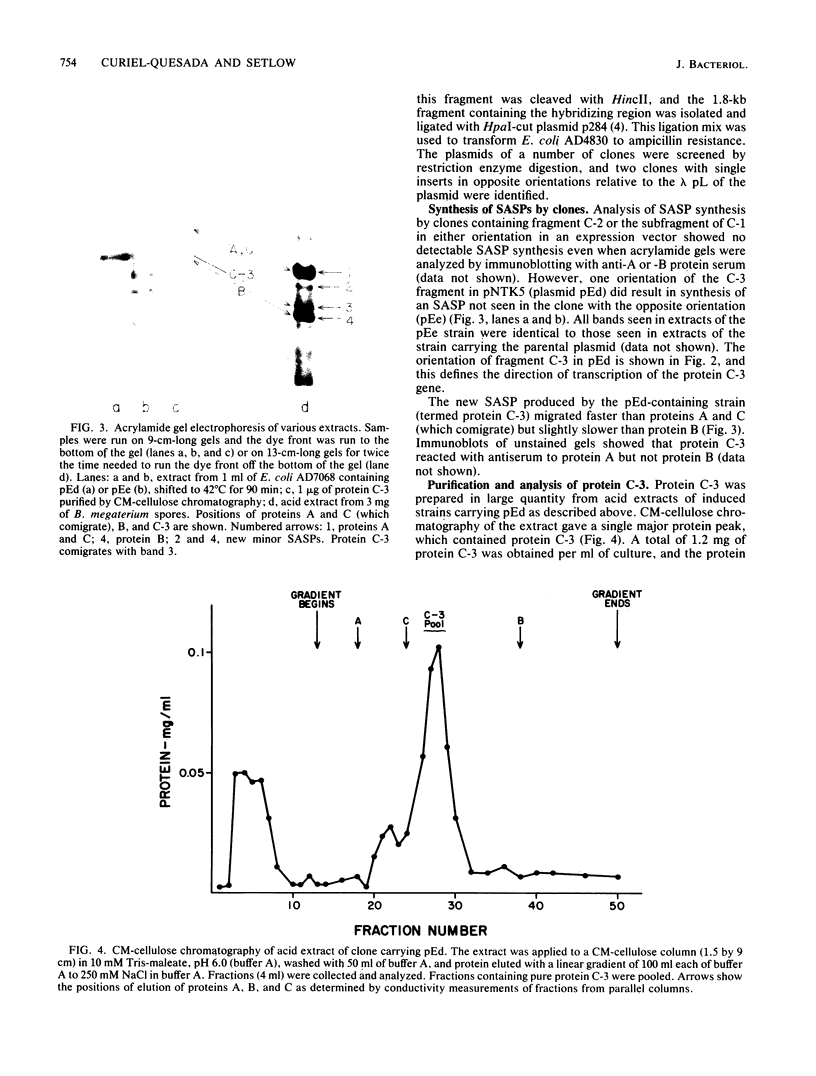
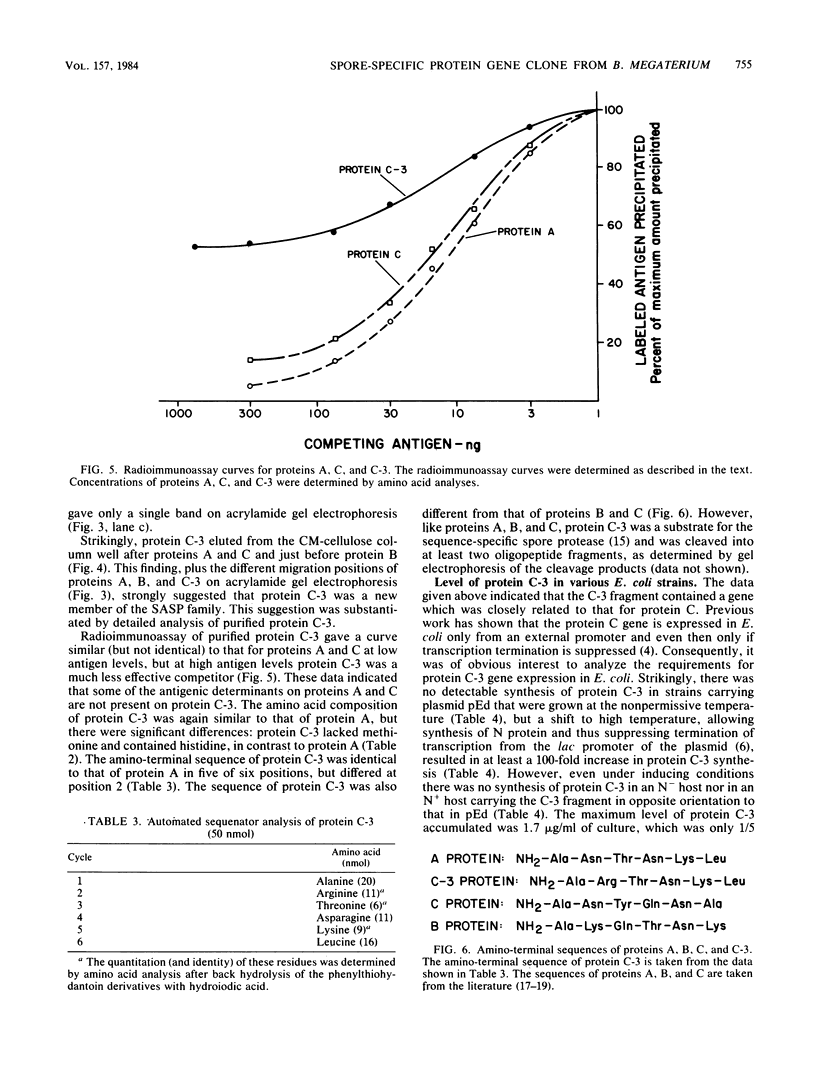
Three EcoRI fragments of Bacillus megaterium DNA hybridized only under nonrestrictive conditions on Southern blots to a probe containing the previously cloned gene for protein C, a small, acid-soluble spore protein (SASP) from B. megaterium. All three fragments were cloned in Escherichia coli cells in plasmid pBR325, and after being transferred to an E. coli expression vector, one of the fragments (C-3) directed the synthesis of a new small, acid-soluble spore protein (termed C-3) immunologically related to protein C. As previously observed with the protein C gene, protein C-3 gene expression in E. coli required an external promoter and suppression of termination of transcription. Protein C-3 was purified from induced E. coli cells, and its immunological properties, electrophoretic mobility, amino acid composition, and amino-terminal sequence were determined. These data indicated that protein C-3 was related, but not identical, to either protein C or the closely related protein A--two of the major small, acid-soluble spore proteins of B. megaterium. Detailed examination of acid extracts of B. megaterium spores showed that they contained a minor protein which comigrated with C-3 on acrylamide gel electrophoresis at low pH and reacted immunologically like C-3.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beltz G. A., Jacobs K. A., Eickbush T. H., Cherbas P. T., Kafatos F. C. Isolation of multigene families and determination of homologies by filter hybridization methods. Methods Enzymol. 1983;100:266–285. doi: 10.1016/0076-6879(83)00061-0. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Curiel-Quesada E., Setlow B., Setlow P. Cloning of the gene for C protein, a low molecular weight spore-specific protein from Bacillus megaterium. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3250–3254. doi: 10.1073/pnas.80.11.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Goldrick S., Setlow P. Expression of a Bacillus megaterium sporulation-specific gene during sporulation of Bacillus subtilis. J Bacteriol. 1983 Sep;155(3):1459–1462. doi: 10.1128/jb.155.3.1459-1462.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Localization of low-molecular-weight basic proteins in Bacillus megaterium spores by cross-linking with ultraviolet light. J Bacteriol. 1979 Aug;139(2):486–494. doi: 10.1128/jb.139.2.486-494.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XVII. Sulfhydryl and disulfide levels in dormancy and germination. J Bacteriol. 1969 Dec;100(3):1155–1160. doi: 10.1128/jb.100.3.1155-1160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Ozols J. Covalent structure of protein A. A low molecular weight protein degraded during germination of Bacillus megaterium spores. J Biol Chem. 1979 Dec 10;254(23):11938–11942. [PubMed] [Google Scholar]
- Setlow P., Ozols J. Covalent structure of protein C. A second major low molecular weight protein degraded during germination of Bacillus megaterium spores. J Biol Chem. 1980 Sep 25;255(18):8413–8416. [PubMed] [Google Scholar]
- Setlow P., Ozols J. The complete covalent structure of protein B. The third major protein degraded during germination of Bacillus megaterium spores. J Biol Chem. 1980 Nov 10;255(21):10445–10450. [PubMed] [Google Scholar]
- Setlow P. Purification and characterization of additional low-molecular-weight basic proteins degraded during germination of Bacillus megaterium spores. J Bacteriol. 1978 Oct;136(1):331–340. doi: 10.1128/jb.136.1.331-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Purification and properties of a specific proteolytic enzyme present in spores of Bacillus magaterium. J Biol Chem. 1976 Dec 25;251(24):7853–7862. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasantha N., Uratani B., Ramaley R. F., Freese E. Isolation of a developmental gene of Bacillus subtilis and its expression in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Feb;80(3):785–789. doi: 10.1073/pnas.80.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H. C., Schnepf H. E., Whiteley H. R. Transcriptional and translational start sites for the Bacillus thuringiensis crystal protein gene. J Biol Chem. 1983 Feb 10;258(3):1960–1967. [PubMed] [Google Scholar]