Abstract
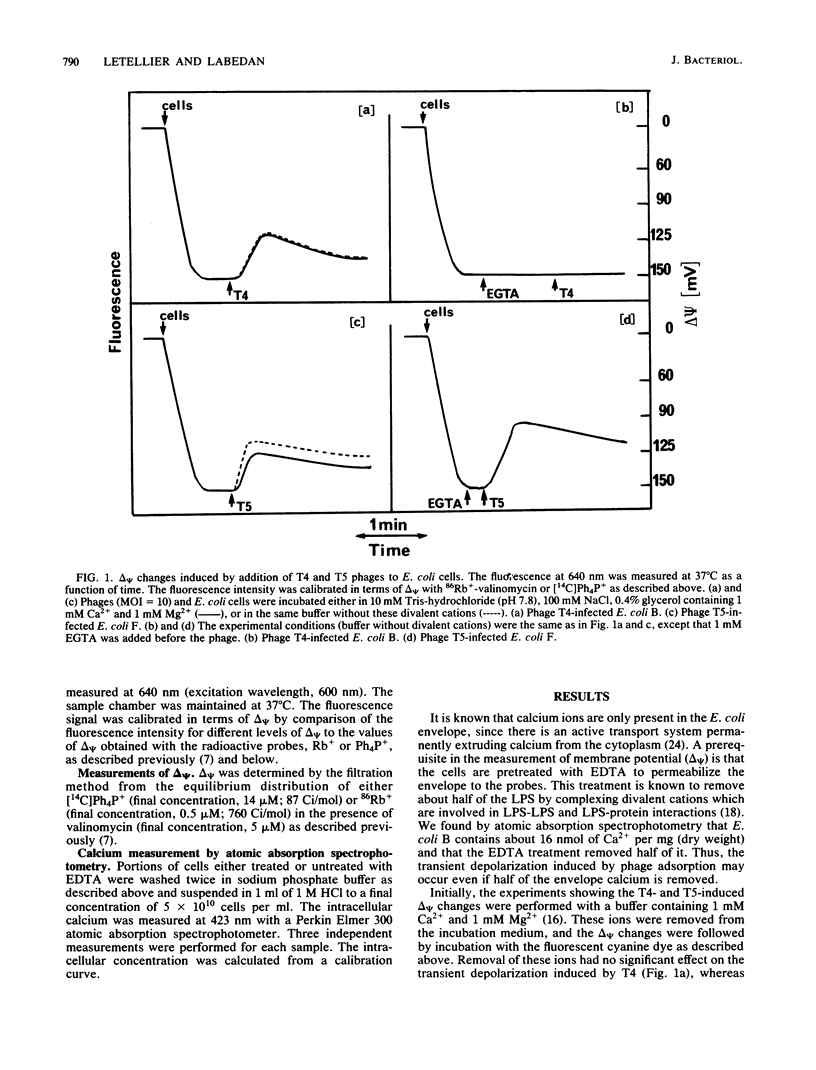
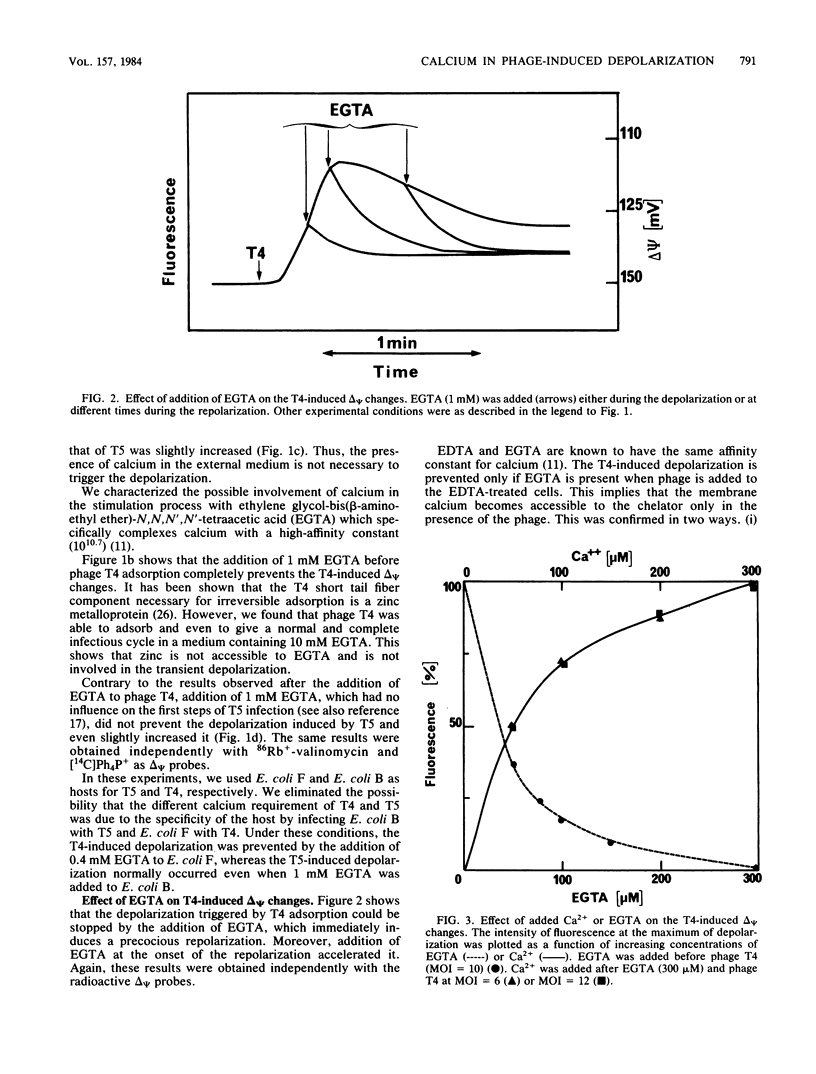
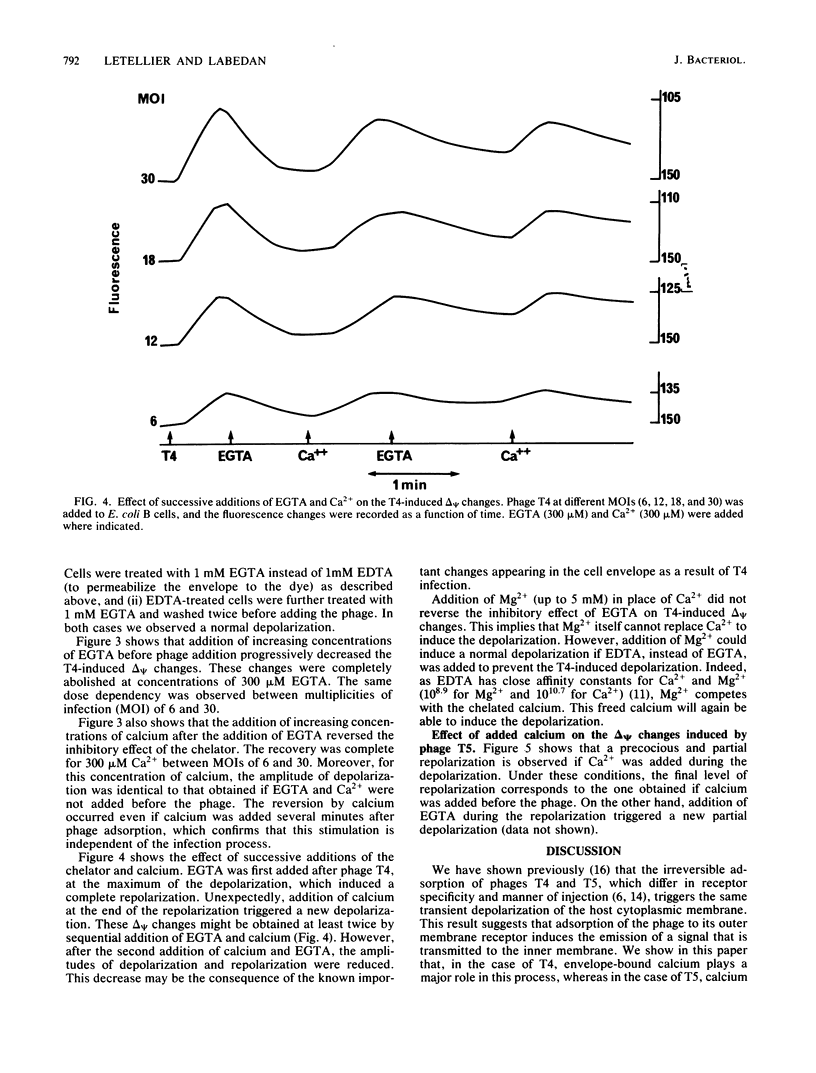
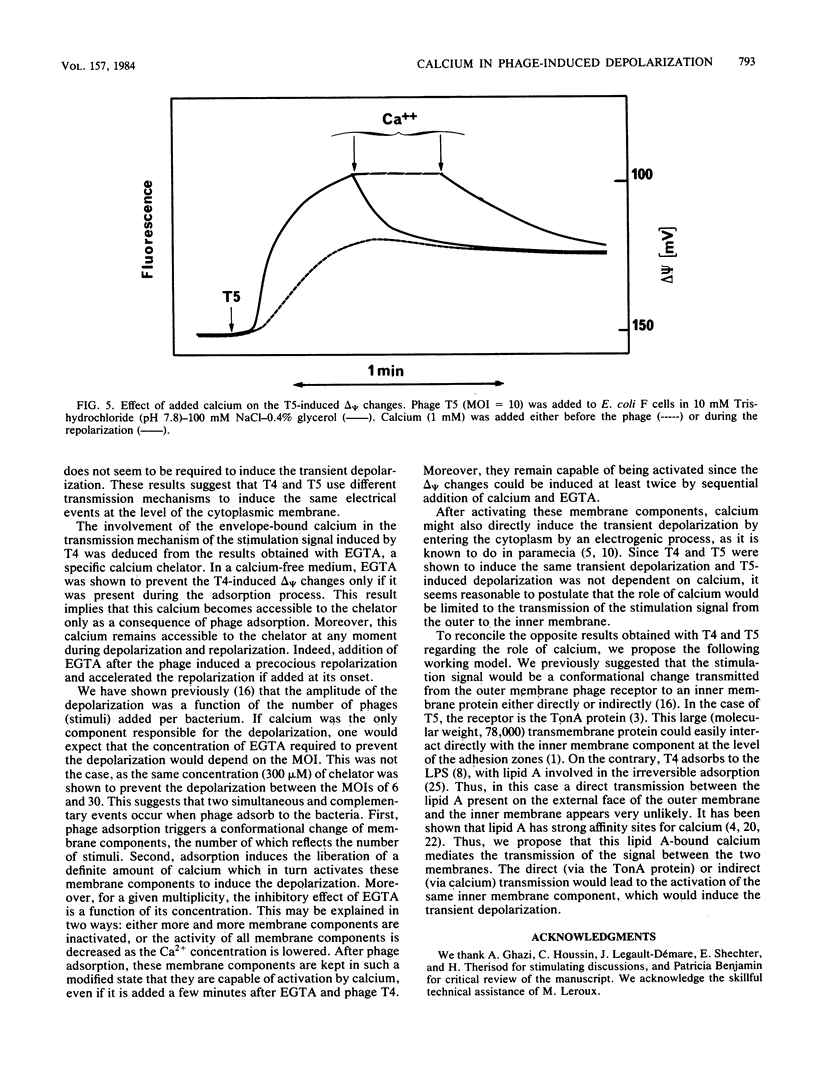
We previously showed that adsorption of bacteriophages T4 and T5 to their respective outer membrane receptors induced a partial depolarization of the cytoplasmic membrane. As these membrane potential changes were independent of phage properties, we proposed that phage adsorption triggered the emission of a signal which must be transmitted between the two membranes. We show here that these two phages use different mechanisms of transmission of this stimulation signal. In the case of T4, but not of T5, a specific requirement for envelope-bound calcium was found. Indeed, addition of ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid prevented the membrane potential changes induced by T4. This envelope-bound calcium became accessible to the chelator only as a consequence of phage adsorption and remained in this state during the depolarization and repolarization. Membrane potential changes again occurred if calcium was added after the addition of ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid and phage. The same concentration (300 microM) of ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid prevented the T4-induced depolarization between multiplicities of infection of 6 and 30. This suggests that phage adsorption triggers both a conformational change of membrane components, the number of which reflects the number of stimuli (phages), and the liberation of a definite amount of calcium. This liberated calcium would, in turn, activate these modified membrane components to induce the depolarization. The fact that depolarization may be induced several times after a unique adsorption implies that these membrane components remain irreversibly modified.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E., Bayer M. H. Fast responses of bacterial membranes to virus adsorption: a fluorescence study. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5618–5622. doi: 10.1073/pnas.78.9.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Schaller K., Wolff H. A common receptor protein for phage T5 and colicin M in the outer membrane of Escherichia coli B. Biochim Biophys Acta. 1973 Sep 27;323(1):87–97. doi: 10.1016/0005-2736(73)90433-1. [DOI] [PubMed] [Google Scholar]
- Coughlin R. T., Tonsager S., McGroarty E. J. Quantitation of metal cations bound to membranes and extracted lipopolysaccharide of Escherichia coli. Biochemistry. 1983 Apr 12;22(8):2002–2007. doi: 10.1021/bi00277a041. [DOI] [PubMed] [Google Scholar]
- Eckert R., Brehm P. Ionic mechanisms of excitation in Paramecium. Annu Rev Biophys Bioeng. 1979;8:353–383. doi: 10.1146/annurev.bb.08.060179.002033. [DOI] [PubMed] [Google Scholar]
- Filali Maltouf A., Labedan B. Host cell metabolic energy is not required for injection of bacteriophage T5 DNA. J Bacteriol. 1983 Jan;153(1):124–133. doi: 10.1128/jb.153.1.124-133.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazi A., Schechter E., Letellier L., Labedan B. Probes of membrane potential in Escherichia coli cells. FEBS Lett. 1981 Mar 23;125(2):197–200. doi: 10.1016/0014-5793(81)80717-x. [DOI] [PubMed] [Google Scholar]
- Hantke K., Braun V. Fluorescence studies on first steps of phage-host interactions. Virology. 1974 Mar;58(1):310–312. doi: 10.1016/0042-6822(74)90167-6. [DOI] [PubMed] [Google Scholar]
- Labedan B., Goldberg E. B. Requirement for membrane potential in injection of phage T4 DNA. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4669–4673. doi: 10.1073/pnas.76.9.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labedan B. In vitro study of the phage T5 DNA injection process: use of columns of Escherichia coli membranes immobilized on kieselguhr. Virology. 1978 Apr;85(2):487–493. doi: 10.1016/0042-6822(78)90455-5. [DOI] [PubMed] [Google Scholar]
- Labedan B., Legault-Demare J. Evidence for heterogeneity in populations of T5 bacteriophage. J Virol. 1974 May;13(5):1093–1100. doi: 10.1128/jvi.13.5.1093-1100.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labedan B., Letellier L. Membrane potential changes during the first steps of coliphage infection. Proc Natl Acad Sci U S A. 1981 Jan;78(1):215–219. doi: 10.1073/pnas.78.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni Y. T. First-step-transfer deoxyribonucleic acid of bacteriophage T5. Bacteriol Rev. 1968 Sep;32(3):227–242. doi: 10.1128/br.32.3.227-242.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letellier L., Shechter E. Cyanine dye as monitor of membrane potentials in Escherichia coli cells and membrane vesicles. Eur J Biochem. 1979 Dec 17;102(2):441–447. doi: 10.1111/j.1432-1033.1979.tb04259.x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Schindler M., Osborn M. J. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry. 1979 Oct 2;18(20):4425–4430. doi: 10.1021/bi00587a024. [DOI] [PubMed] [Google Scholar]
- Tsujibo H., Rosen B. P. Energetics of calcium efflux from cells of Escherichia coli. J Bacteriol. 1983 May;154(2):854–858. doi: 10.1128/jb.154.2.854-858.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzopulos J., Delong S., Chapman V., Kozloff L. M. Host receptor site for the short tail fibers of bacteriophage T4D. Virology. 1982 Jul 15;120(1):33–41. doi: 10.1016/0042-6822(82)90004-6. [DOI] [PubMed] [Google Scholar]



