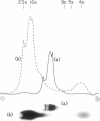
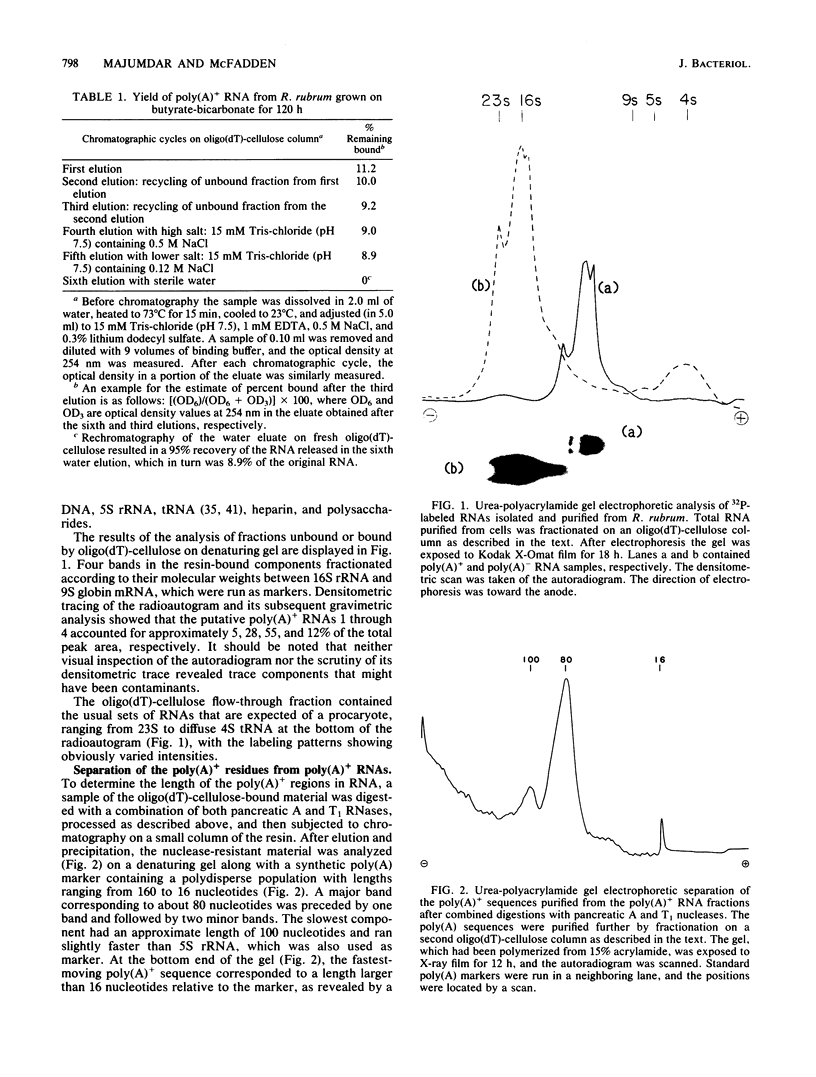
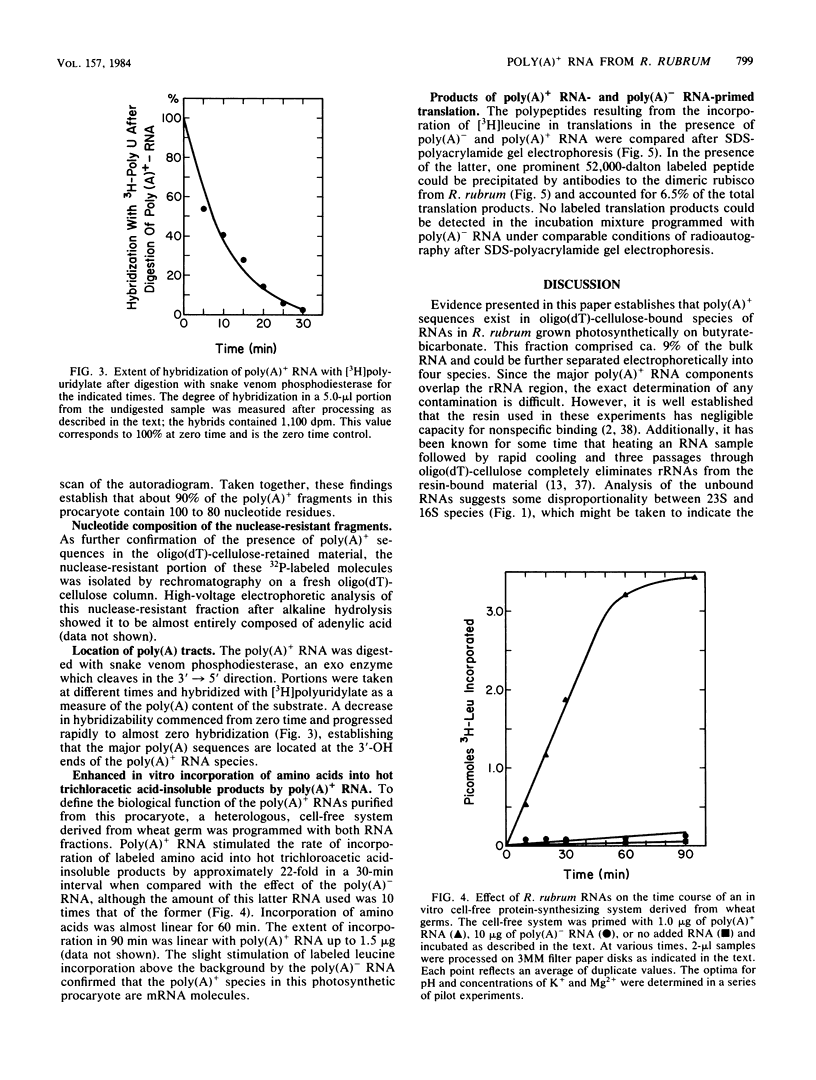
Abstract
Total cellular RNA extracted from Rhodospirillum rubrum cultured in butyrate-containing medium under strict photosynthetic conditions to the stationary phase of growth has been fractionated on an oligodeoxythymidylic acid-cellulose column into polyadenylated [poly(A)+] RNA and poly(A)- RNA fractions. The poly(A)+ fraction was 9 to 10% of the total bulk RNA isolated. Analysis of the poly(A)+ RNA on a denaturing urea-polyacrylamide gel revealed four sharp bands of RNA distributed in heterodisperse fashion between 16S and 9S. Similar fractionation of the poly(A)- RNA resulted in the separation of 23, 16, and 5S rRNAs and 4S tRNA. Poly(A)+ fragments isolated after combined digestion with pancreatic A and T1 RNases and analysis by denaturing gel electrophoresis demonstrated two major components of 80 and 100 residues. Alkaline hydrolysis of the nuclease-resistant, purified residues showed AMP-rich nucleotides. Through the use of snake venom phosphodiesterase, poly(A) tracts were placed at the 3' end of poly(A)+ RNA. Stimulation of [3H]leucine incorporation into hot trichloroacetic acid-precipitable polypeptides in a cell-free system from wheat germ primed by the poly(A)+ RNA mixture was found to be 220-fold higher than that for poly(A)- RNAs (on a unit mass basis), a finding which demonstrated that poly(A)+ RNAs in R. rubrum are mRNAs. Gel electrophoretic analysis of the translation mixture revealed numerous 3H-labeled products including a major band (Mr, 52,000). The parent protein was precipitated by antibodies to ribulose bisphosphate carboxylase-oxygenase and comprised 6.5% of the total translation products.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. D., Adams M. J., Rossmann M. G., Goldenberg E. Letter: A crystalline form of testes-specific lactate dehydrogenase. J Mol Biol. 1973 Aug 25;78(4):721–722. doi: 10.1016/0022-2836(73)90292-1. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Brawerman G. Eukaryotic messenger RNA. Annu Rev Biochem. 1974;43(0):621–642. doi: 10.1146/annurev.bi.43.070174.003201. [DOI] [PubMed] [Google Scholar]
- Broglie R., Bellemare G., Bartlett S. G., Chua N. H., Cashmore A. R. Cloned DNA sequences complementary to mRNAs encoding precursors to the small subunit of ribulose-1,5-bisphosphate carboxylase and a chlorophyll a/b binding polypeptide. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7304–7308. doi: 10.1073/pnas.78.12.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. H., Majumdar A., Dunn R., Makabe O., RajBhandary U. L., Khorana H. G., Ohtsuka E., Tanaka T., Taniyama Y. O., Ikehara M. Bacteriorhodopsin: partial sequence of mRNA provides amino acid sequence in the precursor region. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3398–3402. doi: 10.1073/pnas.78.6.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C. T. Properties of ribonucleic acids from photosynthetic and heterotrophic Rhodospirillum rubrum. Can J Microbiol. 1976 Feb;22(2):228–236. doi: 10.1139/m76-031. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F. Postmaturational cleavage of 23s ribosomal ribonucleic acid and its metabolic control in the blue-green alga Anacystis nidulans. J Bacteriol. 1973 Mar;113(3):1256–1263. doi: 10.1128/jb.113.3.1256-1263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Kopp D. W. The occurrence of polyadenylate sequences in bacteria and yeast. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1531–1537. doi: 10.1016/0006-291x(70)90561-9. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna Y., Sarkar N. Characterization of polyadenylate-containing ribonucleic acid from Bacillus subtilis. Biochemistry. 1982 May 25;21(11):2724–2729. doi: 10.1021/bi00540a023. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna Y., Sarkar N. Selective resistance of bacterial polyadenylate-containing RNA to hydrolysis by guanosine 3'-5'-monophosphate-sensitive nuclease of Bacillus brevis. Biochem Biophys Res Commun. 1981 Nov 30;103(2):454–460. doi: 10.1016/0006-291x(81)90474-5. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna Y., Sarkar N. The synthesis of DNA complementary to polyadenylate-containing RNA from Bacillus subtilis. J Biol Chem. 1982 Mar 25;257(6):2747–2750. [PubMed] [Google Scholar]
- Gorski J., Morrison M. R., Merkel C. G., Lingrel J. B. Size heterogeneity of polyadenylate sequences in mouse globin messenger RNA. J Mol Biol. 1974 Jun 25;86(2):363–371. doi: 10.1016/0022-2836(74)90025-4. [DOI] [PubMed] [Google Scholar]
- HORIUCHI T., HORIUCHI S., MIZUNO D. Degradation of ribonucleic acid in Escherichia coli in phosphorus-deficient culture. Biochim Biophys Acta. 1959 Feb;31(2):570–572. doi: 10.1016/0006-3002(59)90044-7. [DOI] [PubMed] [Google Scholar]
- Holland M. J., Holland J. P. Isolation and identification of yeast messenger ribonucleic acids coding for enolase, glyceraldehyde-3-phosphate dehydrogenase, and phosphoglycerate kinase. Biochemistry. 1978 Nov 14;17(23):4900–4907. doi: 10.1021/bi00616a007. [DOI] [PubMed] [Google Scholar]
- Kerjan P., Szulmajster J. Isolation and characterization of polyadenylated RNA species from sporulating cells of Bacillus subtilis. Biochem Biophys Res Commun. 1980 Mar 13;93(1):201–208. doi: 10.1016/s0006-291x(80)80266-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Levy S. B. Very stable prokaryotic messenger RNA in chromosomeless Escherichia coli minicells. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2900–2904. doi: 10.1073/pnas.72.8.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar P. K., Vipparti V. A. Polyadenylated messenger RNAs code for photo reaction center and light-harvesting antenna polypeptides of Rhodospirillum rubrum. FEBS Lett. 1980 Jan 1;109(1):31–33. doi: 10.1016/0014-5793(80)81304-4. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Mansour J. D., Stachow C. S. Structural changes in the ribosomes and ribosomal proteins of Rhodopseudomonas palustris. Biochem Biophys Res Commun. 1975 Jan 20;62(2):276–281. doi: 10.1016/s0006-291x(75)80134-3. [DOI] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato H., Venkatesan S., Edmonds M. Polyadenylic acid sequences in E. coli messenger RNA. Nature. 1975 Jul 10;256(5513):144–146. doi: 10.1038/256144a0. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Zusman D. R. Evidence for long-lived mRNA during fruiting body formation in myxococcus xanthus. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1467–1471. doi: 10.1073/pnas.80.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta N., Sanders M., Newton A. Characterization of unstable poly (A)-RNA in Caulobacter crescentus. Biochim Biophys Acta. 1978 Jan 26;517(1):65–75. doi: 10.1016/0005-2787(78)90034-5. [DOI] [PubMed] [Google Scholar]
- Palatnik C. M., Storti R. V., Capone A. K., Jacobson A. Messenger RNA stability in Dictyostelium discoideum: does poly(A) have a regulatory role? J Mol Biol. 1980 Aug 5;141(2):99–118. doi: 10.1016/0022-2836(80)90379-4. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., LaTorre J. Lack of polyadenylic acid sequences in the messenger RNA of E. coli. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1593–1600. doi: 10.1016/0006-291x(72)90896-0. [DOI] [PubMed] [Google Scholar]
- Sarkar N., Langley D., Paulus H. Isolation and characterization of polyadenylate-containing RNA from Bacillus brevis. Biochemistry. 1978 Aug 22;17(17):3468–3474. doi: 10.1021/bi00610a007. [DOI] [PubMed] [Google Scholar]
- Sarles L. S., Tabita F. R. Derepression of the synthesis of D-ribulose 1,5-bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum. J Bacteriol. 1983 Jan;153(1):458–464. doi: 10.1128/jb.153.1.458-464.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scragg A. H., Thurston C. F. Characterization and messenger activity of poly(A)-containing RNA from Chlorella. J Gen Microbiol. 1975 Jul;89(1):155–162. doi: 10.1099/00221287-89-1-155. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Darnell J. E. Polyadenylic acid segment in mRNA becomes shorter with age. Nat New Biol. 1973 Feb 28;241(113):265–268. doi: 10.1038/newbio241265a0. [DOI] [PubMed] [Google Scholar]
- Srinivasan P. R., Ramanarayanan M., Rabbani E. Presence of polyriboadenylate sequences in pulse-labeled RNA of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2910–2914. doi: 10.1073/pnas.72.8.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A. D-ribulose 1,5-diphosphate carboxylase from Rhodospirillum rubrum. I. Levels, purification, and effects of metallic ions. J Biol Chem. 1974 Jun 10;249(11):3453–3458. [PubMed] [Google Scholar]
- Verma D. P., Nash D. T., Schulman H. M. Isolation and in vitro translation of soybean leghaemoglobin mRNA. Nature. 1974 Sep 6;251(5470):74–77. doi: 10.1038/251074a0. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Firtel R. A., Lodish H. F., Baltimore D. Synthesis of DNA complementary to cellular slime mold messenger RNA by reverse transcriptase. Biochemistry. 1974 Sep 10;13(19):3917–3922. doi: 10.1021/bi00716a016. [DOI] [PubMed] [Google Scholar]
- Watanabe A., Price C. A. Translation of mRNAs for subunits of chloroplast coupling factor 1 in spinach. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6304–6308. doi: 10.1073/pnas.79.20.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin S. S., Gibson K. D. Changes in ribonucleic acid turnover during aerobic and anaerobic growth in Rhodopseudomonas spheroides. J Bacteriol. 1972 May;110(2):677–683. doi: 10.1128/jb.110.2.677-683.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita J., Kamen M. D. Observations on the nature of pulse-labeled RNA's from photosynthetically or heterotrophically grown Rhodospirillum rubrum. Biochim Biophys Acta. 1968 Jun 18;161(1):162–169. doi: 10.1016/0005-2787(68)90305-5. [DOI] [PubMed] [Google Scholar]