Abstract
In this paper a new atomic force sensing technique is presented for dynamically probing conformational changes in proteins. The method is applied to the light-induced changes in the membrane-bound proton pump bacteriorhodopsin (bR). The microsecond time-resolution of the method, as presently implemented, covers many of the intermediates of the bR photocycle which is well characterized by spectroscopical methods. In addition to the native pigment, we have studied bR proteins substituted with chemically modified retinal chromophores. These synthetic chromophores were designed to restrict their ability to isomerize, while maintaining the basic characteristic of a large light-induced charge redistribution in the vertically excited Franck–Condon state. An analysis of the atomic force sensing signals lead us to conclude that protein conformational changes in bR can be initiated as a result of a light-triggered redistribution of electronic charge in the retinal chromophore, even when isomerization cannot take place. Although the coupling mechanism of such changes to the light-induced proton pump is still not established, our data question the current working hypothesis which attributes all primary events in retinal proteins to an initial trans⇔cis isomerization.
The function of light-sensitive retinylidene proteins, such as visual pigments and bacteriorhodopsin (bR), is determined by protein structural changes, which are induced by the absorption of a photon (for a recent series of reviews, see ref. 1). It is widely accepted that all light-induced protein conformational alterations in retinylidene proteins are initiated by isomerization of the retinal chromophore: 11-cis → trans (in visual pigments) or trans → 13-cis (in bR). Nonetheless, alternative mechanisms have been suggested in which isomerization is not the primary trigger of the protein structural alterations (2–5). One approach is based on the suggestion that a large light-induced redistribution of electronic charge in the vertically excited state causes alterations in the surrounding protein matrix and leads to the subsequent protein structural transformations (3). In support of this mechanism, there is unequivocal evidence based on nonlinear optics data (6–8) that, with similarity to retinals in solution (9), light excitation of bR does indeed initiate a large light-induced electron redistribution in the polyene. However, the connection between this charge redistribution and protein conformational changes has remained elusive. In this paper we approach this problem by means of a new methodology which directly monitors protein conformational changes using an atomic force probe.
The new methodology evolves from a recently presented technique, based on atomic force sensing (AFS), which monitored protein conformational changes induced in bR by steady-state illumination (10). In the present work, we extend the AFS technique to dynamically probe protein conformational changes with microsecond time resolution. The method is applied to the light-driven proton pump, bR, which is found in the purple membrane of Halobacterium salinarium (11). The cyclic protein conformational changes associated with this pump are initiated by light absorption by the all-trans retinylidene chromophore, which is bound to the protein via a protonated Schiff base linkage. The resulting cross-membrane proton translocation involves complex structural changes in the chromophore and in the surrounding protein which have been extensively investigated using a variety of spectrosocopic methods (1). The time-resolved AFS methodology provides a new tool for studying the bR photocycle. In the present work it is applied to native bR as well as to several artificial bR pigments in which the critical C13=C14 bond is blocked by appropriate ring structures. The data show that in a retinylidene protein, such as bR, protein conformational changes can be initiated by the large electronic charge redistribution induced in the chromophore by the absorption of a photon.
MATERIALS AND METHODS
A suspension at pH 7 of purple membrane fragments containing bR was prepared by the standard method of Oesterhelt and Stoeckenius (12). The films were prepared by drying a purple membrane suspension onto a glass slide using a mild vacuum (≈15 mmHg). The artificial pigments derived from retinal analogs were prepared by incubating the respective aldehyde chromophores at room temperature with the apomembrane (13). The reduced protonated Schiff base analogs were obtained by reducing the pigments with sodium borohydride (14). All artificial pigment films were prepared similarly to those of native bR.
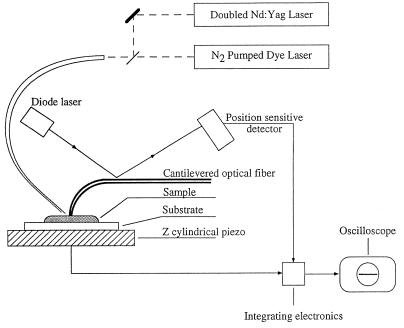
Schematic representation of the apparatus used for sensing light-induced transformations in bR with an atomic force sensor is shown in Fig. 1. The deflection of the cantilever is monitored by reflecting a diode laser in the near-infrared off a metalized portion of the cantilever and onto a position-sensitive detector, which has four quadrants. The time-response of the electrical signals from this detector are monitored directly with a digitizing oscilloscope (Tektronix model 2430). The time response of the apparatus is ≈2 μsec and is limited by the electronic analysis of the signals emanating from the position sensitive detector. The oscilloscope is synchronized with laser pulses from a JK Nd:YAG (Nd:yttrium/aluminum garnet) laser that is doubled to produce a 532 nm emission. Alternatively, the sample was illuminated with a PRA 1000 N2/dye laser system. An appropriate delay system allows firing the lasers consecutively with a delay that can be varied starting from 1 μsec. Both lasers were transmitted through the same optical fiber that directs the light onto the same region of the sample, which is sensed by the cantilever. The cantilevers we found to be appropriate for these measurements were formed out of drawn glass fibers that were developed for near-field optical microscopy (15, 16). These glass structures permit great flexibility of the tip that allows it to respond with very high frequencies. Furthermore, the nature of these cantilevered probes is such that the normal modes significantly couple the tip to the cantilever, and thus any small motion of the tip is reflected in the cantilever position. The standard characteristics of the tips that we used had a force constant of ≈400 N/m and a resonance frequency between 300 and 400 kHz. These properties do not apply to standard silicon cantilevers in which there is little tip flexibility and essentially no coupling between the tip and the cantilever.
Figure 1.
Schematic representation of the experimental system as described in the text.
RESULTS AND DISCUSSION
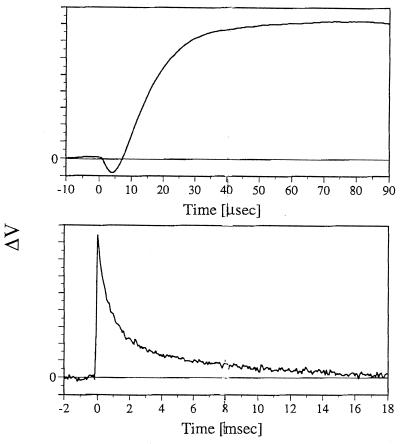
In atomic force microscopy (AFM) a tip, at the end of a cantilever, which is ≈20 nm in diameter, is brought onto a surface and is scanned across the surface to form a topographical image (17). The AFS experiments reported in this work are based on using the AFM in a resting (nonscanning) mode, similar to the approach recently applied by Hansma and coworkers (18) in their investigation of enzymatic digestion. In Fig. 2, we show the response of such a resting cantilever, which is in contact with a preparation of bR membrane layers, to 0.5–5 nsec laser pulses, which are absorbed by the bR molecules. The first signal that is seen above the ≈2-μsec time response of the electronics is a decrease in the Z position of the cantilever which is maximal at ≈8 μsec. The subsequent change in the signal indicates an expansion of the membrane detected as a positive response of the cantilever, which is maximal at ≈35 μsec. Finally, the positive signal decays with a biphasic response and reaches the baseline noise level ≈16 msec from the incidence of the light pulse. Although occurring in the same time range the above kinetic patterns cannot be simply related to those of the bR photocycle intermediates. Thus, the growing-in and the slow (≈5 msec) decay component of the AFS signals are in qualitative agreement with the rise and decay of the M intermediate which plays a key role in the pump mechanism. However, the first (≈0.5 msec) component of the AFS decay is considerably faster than the M decay.
Figure 2.
Changes in the voltage output of the position sensitive detector observed, over two time ranges, following 532 nm laser excitation of an unoriented bR film. Films were prepared by drying a bR suspension on a glass slide using a mild vacuum (≈15 mmHg). All measurements were carried out under 100% humidity.
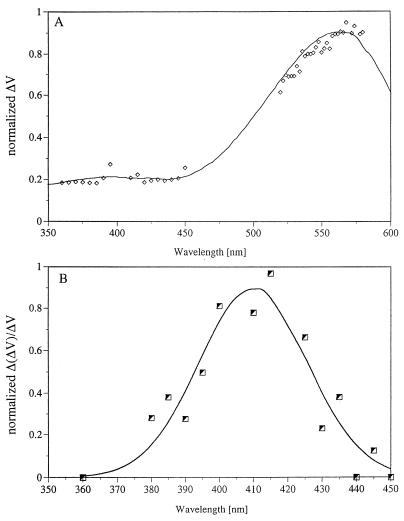
In keeping with the agreement in the time scales of the photocycle, additional evidence indicates that the kinetic cantilever response is directly associated with protein structural alterations induced by light absorption. First, as shown in Fig. 3A, the action spectrum of the AFS signal closely coincides with the absorption spectrum of bR. In addition, double pulse AFS experiments clearly tie the AFS experiments to bR photocycle photon absorption. In the experiments the first (532 nm) laser pulse initiates the bR photocycle, while the second (410 nm) is appropriately delayed (≈300 μsec) so as to be absorbed by the characteristic M intermediate (λmax = 410 nm). The second pulse was found to reverse the AFS signal generated by the first pulse, in keeping with the well-documented photoreaction of M, back to the initial bR pigment (19–21). The action spectrum of the net AFS signal due to the second pulse, shown in Fig. 3B, clearly coincides with the well-documented absorption spectrum of M. This indicates that both (first and second) AFS transformations are coupled to the bR photocycle.
Figure 3.
(A) The AFS light-induced action spectrum of a bR film obtained using (tunable) dye laser excitation. The solid line represents the measured absorption spectrum, while the points represent alterations in the position sensitive detector voltage (PSDV) as a function of the excitation wavelength (normalized to the variation in pulse intensity). (B) Action spectrum of the AFS back-photoreaction monitored in a two-pulse experiment. The first laser (532 nm) drives the bR photocycle, while the second (tunable dye) laser pulse is fired with a delay of 100 μsec. The points represent the net effect of the second laser after correcting the effects due to its absorption by bR molecules, which have not been photocycled by the first pulse. The net effect of the second pulse, denoted as Δ (ΔPSDV), is to reverse the AFS signal. The points represent this signal reversal as a function of the dye laser wavelength. The solid line, representing the best fit curve, closely coincides with the absorption spectrum of the M intermediate.
The fact that the AFS signals are coupled to the photocycle indicates that they are not associated with significant sample heating artifacts. A similar conclusion can be derived by comparing the relative size of the signals obtained upon excitation at the maximum of the main bR absorption band (570 nm) and those obtained when the excitation wavelength is at, for example, 360 nm, where the pigment extinction is lower by a factor of ≈5. As shown in Fig. 3A, the relative magnitude of the observed AFS signals is well correlated with this extinction ratio. Since the 360 and 570 nm photons differ in energy by a factor of 1.6, it is evident that heating effects do not significantly contribute to the signal. Furthermore, a series of control experiments were performed in which β-carotene (λmax = 450 nm), anthracene (λmax = 360 nm), and excess retinal (λmax = 380 nm) were incorporated into the purple membrane or into its apomembrane. In each case, excitation with the appropriate wavelength did not reveal an AFS response as a result of light absorption by the respective chromophores. We should also point out that besides heating effects, artifacts that could affect the cantilever position, such as light-induced alterations in membrane electrostatics, could also be excluded. Thus, glass cantilevers that were coated with metal gave signals analogous to those observed with uncoated cantilevers. One should finally mention that although assigned to light-induced conformational changes in the protein, our AFS signals may include contributions of changes in the lipid region of the membrane. The latter being induced by primary changes in the conformation of the protein. Interestingly in this respect, preliminary experiments in our laboratory show light-induced AFS signals with a nonmembrane protein such as the “Yellow Protein” (22).
It is evident that the above AFS patterns, which are interpreted in terms of light-induced protein conformational changes, should be correlated with methods (23–27) that have been used for monitoring light-induced volume changes in bR. Such methods include time-resolved photoacoustic responses (24), as well as photothermal beam deflection (26) and the analysis of pressure effects on the photocycle kinetics (28). Our data qualitatively agree with a volume contraction at the stage of the L precursor of M and with a volume expansion at the M stage (26, 27). It is obvious, however, that further work will have to be carried out to allow a detailed comparison between the various methodologies. For example, in variance with the above experiments, our AFS data indicate that the decay of the positive (expansion) signal does not exactly match the decay of the spectrosocopically defined M intermediate.
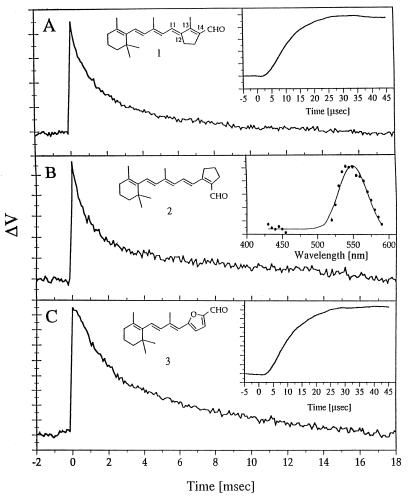
As mentioned above, a major goal of the present work was to address the nature of the primary events of the bR photocycle. We approached this issue by combining the light-induced AFS technique with the well-established methodology of replacing the native retinylidene chromophore of bR with synthetic analogs (for a review, see ref. 29). The specific choices were artificial pigments (I, II, and III) formed from three locked retinal analogs 1,2 (30) and 3 (31, 32), in which the critical C13=C14 isomerization is blocked by a 5-membered ring structure. Although pigment I shows transient absorbance and fluorescence changes on a picosecond time scale (4), all three pigments do not exhibit any of the microsecond–millisecond changes in the chromophore absorption spectrum, which characterize the photocycle of native bR (4, 28, 32, 33). However, surprisingly, each of the three pigments was found to exhibit light-induced AFS patterns. As confirmed in the case of pigment II (Fig. 4B Inset), the AFS action spectrum again closely coincides with the absorption spectrum, which (for pigment II) is blue shifted by ≈15 nm with respect to native bR.
Figure 4.
Observed alterations in the position sensitive detector voltage as a result of 532 nm excitation of artificial bR films (at 100% humidity) derived from the synthetic aldehydes, 1, 2, and 3. (A) Pigment I. (B) Pigment II. The insert represents the action spectrum of the AFS signal, obtained as described in Fig. 3. (C) Pigment III.
One is therefore faced with the challenge of accounting for the AFS patterns and for the related protein structural changes in the C13=C14 locked molecules, without requiring a primary all-trans → 13-cis isomerization. We wish to suggest an approach that is based on the electrostatic event discussed in the Introduction. A fundamental feature common to all retinal proteins (including the synthetic pigments described above) is the large electronic charge redistribution in the vertically excited (Franck Condon) state (6–9). We suggest that such redistribution of charge in the retinal triggers a series of conformational changes in the protein, persisting for several milliseconds.
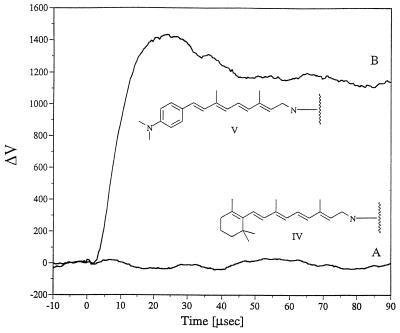
To support this suggestion, we carried out light-induced AFS measurements with a pigment in which the protonated Schiff base, which links the retinal to the protein, was reduced by sodium borohydride (14). In this reduced (symmetric) pigment (IV), a significant electronic charge redistribution in the symmetric chromophore can be excluded. This can be deduced from the fact that a polyene system such as the reduced native bR chromophore should not have a significant dipole in view of the fact that there are no electron withdrawing or electron donating groups on the polyene chain to generate an asymmetric charge distribution upon excitation. To verify this, the light-induced dipoles of the most critical artificial molecules of the present work has recently been investigated using second order nonlinear optical spectroscopies (unpublished data in our laboratory). The reduced pigment (IV) is characterized by a significantly reduced dipole relative to bR. In fact, no light-induced AFS signals were observed (Fig. 5A), despite the fact that this chromophore is capable of photoisomerization in any of its polyene double bonds.
Figure 5.
Observed alterations in the position sensitive detector voltage as a result of tunable dye laser excitation that was suitable for the absorption of the artificial bR films (at 100% humidity) derived from (A) reduced native bR (excitation at 365 nm) and (B) a reduced artificial pigment V (excitation at 395 nm).
To further test this charge redistribution mechanism, we formed an artificial pigment (V) in which the β-ionone ring of the retinal was substituted with a p-dimethylaminobenzene moiety. Pigment V was reduced using the same procedure applied to native bR. In contrast to the reduced native pigment, the reduced, chemically modified pigment exhibited an AFS signal that was similar to that of native bR prior to reduction (Fig. 5B). This observation can be rationalized by assuming that the amino group reinstates a light-induced charge redistribution in the chromophore.
In spite of the feasibility of the light-induced charge redistribution mechanism which does not require isomerization, one should consider an alternative possibility, namely, that the AFS signals observed with the locked pigments are triggered by isomerization around a double bond, other than C13=C14. Such a mechanism is highly unlikely, not because of the behavior of molecule III which excludes C11=C12 as the alternative bond that could isomerize, but primarily since such alternative C=C photoisomerizations would be expected to lead to characteristic (microsecond–millisecond) absorption changes, with analogy to the photocycle of the native system. An additional argument in this respect stems from the fact that the reduced native pigment shows no light-induced AFS signals, despite its capability to undergo C=C photoisomerization.
CONCLUSIONS
In the present work we have demonstrated that AFS, based on AFM technology, may be used for time resolving protein conformational changes with microsecond time resolution. When applied to the light-driven proton pump, bR, the following major conclusions are derived.
(i) In native bR the light-induced AFS signals reflect (still uncharacterized) protein structural changes which are coupled to the (spectroscopic) photocycle intermediates. The AFS kinetics are not identical to those of the photocycle, thus revealing transformations which are silent in absorption spectral measurements.
(ii) Surprisingly, AFS signals and thus protein associated conformational changes, are induced by light also in the case of the C13=C14 locked molecules. It is concluded that such changes are induced via a mechanism that does not involve trans → cis isomerization. We suggest that in such artificial systems the primary photophysical events are driven by the initial polarization of the polyene in the excited Franck–Condon state.
(iii) The question obviously arises as to whether the light-induced AFS signals of native bR reflect structural changes that are analogous to those of the locked pigments. At present this question cannot be answered in a definite way. However, it is tempting to suggest that analogous protein conformational changes are triggered by the light-induced redistribution of polyene electronic charge in the native pigment as well. As shown by the M back-photoreaction experiments such changes become coupled to the spectroscopic photocycle intermediates. In other words, the light-induced conformational changes reflected by the AFS methodology become physiologically meaningful only if a subsequent trans → 13-cis isomerization can take place.
(iv) One should finally address the question as to how does the initially formed polarization in the Franck–Condon state induce protein conformational changes which persist over a millisecond time range. A first requirement is the presence of polar groups in close proximity of the binding site which will respond to the transiently generated polarization along the polyene chain. Plausible candidates are aromatic tryptophan residues which have been shown to stabilize positive charges (34). Bound water molecules should also be considered. Thus, it is accepted that in bR a structured water molecule system is present around the Schiff base linkage (35–40). The induced retinylidene chromophore electronic charge redistribution could alter the water geometry generating an unrelaxed system which in turn will trigger the (AFS) protein structural changes which persist for several millseconds. Such unrelaxed water could also be responsible for the observed light-induced red-shift seen in all such retinylidene proteins.
In conclusion, we have shown the capability of a new time-resolved AFS methodology to monitor, in a direct and simple way, structural changes in a retinal protein which are silent to absorption spectroscopy. More work will be required for identifying the molecular nature of such changes as well as to establish if they do play a role in the bR pump mechanism. A further challenge will be to apply the AFS technique for investigating light-induced conformational alterations in a variety of light-induced bioreactions such as vision and photosynthesis.
Acknowledgments
This work was supported by grants from the Fund for Basic Research (administered by the Israel Academy of Sciences and Humanities) Centers of Excellence Program, by the U.S.–Israel Binational Science Foundation, by the (Germany–Israel) James Franck (Minerva) Program and by the Israeli Ministry for Science and the Arts through its Strategic Infrastructure Grants Program.
ABBREVIATIONS
- bR
bacteriorhodopsin
- AFS
atomic force sensing
- AFM
atomic force microscopy
Note Added in Proof
The results of our work indicate that upon light absorption in the retinylidene chromophore there is a large redistribution of electronic charge. This redistribution of charge occurs in the vertically excited state and is thus instantaneous. Such a charge redistribution in the conformationally restricted retinylidene chromophore results in an ultrafast change in an electrostatically and vibrationally coupled protein coordinate even without isomerization of the retinal chromophore. It is tempting to suggest that this primary sequence of events contributes to the accepted barrierless shape of the excited state potential surface in retinal proteins facilitating cis-trans isomerization. This sequence of events bears analogy to the investigations of the effects of charge redistribution in relatively simple restricted molecular systems that have been reported recently (41). These studies have shown that, as a result of a restriction in the conformational freedom of a simple molecular system, the effect of the charge redistribution is not dispersed in numerous vibrational modes of a conformationally unrestricted molecule. Rather, the effects are confined to particular vibrational modes that result in an alteration in one molecular coordinate that produces a barrierless surface along another major reaction coordinate—i.e., proton transfer in the case of the simple system that has been studied recently.
References
- 1.Ottolenghi, M. & Sheves, M., eds. (1995) Isr. J. Chem. 35, 193–513.
- 2.Salem L, Bruckmann P. Nature (London) 1975;258:526–529. doi: 10.1038/258526a0. [DOI] [PubMed] [Google Scholar]
- 3.Lewis A. Proc Natl Acad Sci USA. 1978;75:543–547. [Google Scholar]
- 4.Delaney J, Brack T, Atkinson G, Ottolenghi M, Steinberg G, Sheves M. Proc Natl Acad Sci USA. 1995;92:2101–2105. doi: 10.1073/pnas.92.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu D, Martin C, Schulten K. Biophys J. 1996;70:453–460. doi: 10.1016/S0006-3495(96)79588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J, Chen Z, Lewis A. J Phys Chem. 1989;93:3314–3320. [Google Scholar]
- 7.Birge R, Zhang X. J Chem Phys. 1990;94:7178–7179. [Google Scholar]
- 8.Clays K, Hendrick E, Triest M, Verbiest T, Persoons A, Dehu C, Bredas J. Science. 1993;262:1419–1422. doi: 10.1126/science.262.5138.1419. [DOI] [PubMed] [Google Scholar]
- 9.Mathies R, Stryer L. Proc Natl Acad Sci USA. 1976;73:2169–2173. doi: 10.1073/pnas.73.7.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis A, Rousso I, Khachatryan E, Brodsky I, Lieberman K, Sheves M. Biophys J. 1996;70:2350–2384. doi: 10.1016/S0006-3495(96)79805-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oesterhelt D, Stoeckenius W. Nature (London) New Biol. 1971;233:149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- 12.Oesterhelt D, Stoeckenius W. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- 13.Sheves M, Friedman N, Albeck A, Ottolenghi M. Biochemistry. 1985;24:1260–1265. [Google Scholar]
- 14.Akhtar M, Blosse P, Dewhurst P. Biochem J. 1968;110:693–698. doi: 10.1042/bj1100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shalom S, Lieberman K, Lewis A, Cohen S. Rev Sci Instrum. 1992;63:061. -.4065. [Google Scholar]
- 16.Lieberman K, Lewis A, Fish G, Jovin T, Schaper A, Cohen S. Appl Phys Lett. 1994;65:648–650. [Google Scholar]
- 17.Binnig G, Quate C, Gerber C. Phys Rev Lett. 1986;56:930–938. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- 18.Radmacher M, Fritz M, Hansma H, Hansma P. Science. 1994;265:1577–1579. doi: 10.1126/science.8079171. [DOI] [PubMed] [Google Scholar]
- 19.Litvin F, Balashov S. Biophysics. 1977;22:1157–1160. [Google Scholar]
- 20.Kalisky O, Lachish U, Ottolenghi M. Photochem Photobiol. 1978;28:261–263. [Google Scholar]
- 21.Druckman S, Friedman N, Lanyi J, Needleman R, Ottolenghi M, Sheves M. Photochem Photobiol. 1992;56:1041–1047. doi: 10.1111/j.1751-1097.1992.tb09727.x. [DOI] [PubMed] [Google Scholar]
- 22.Van Brederode M, Off W, Van Stokkum I, Groot M, Hellingwerf K. Biophys J. 1996;71:365–389. doi: 10.1016/S0006-3495(96)79234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulenberg P, Rohr M, Gartner W, Braslavsky S. Biophys J. 1994;66:838–843. doi: 10.1016/s0006-3495(94)80860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulenberg P, Gartner W, Braslavsky S. J Phys Chem. 1995;99:9617–9625. [Google Scholar]
- 25.Mauzerall D, Gunner R, Zhang J. Biophys J. 1995;68:274–284. doi: 10.1016/S0006-3495(95)80185-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang D, Mauzerall D. Biophys J. 1996;71:381–388. doi: 10.1016/S0006-3495(96)79235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varo G, Lanyi J. Biochemistry. 1995;34:12161–12169. doi: 10.1021/bi00038a009. [DOI] [PubMed] [Google Scholar]
- 28.Chang C, Govindjee R, Ebrey T, Bagley K, Dollinger G, Eisenstein L, Marque J, Rodex H, Vittow J, Fang J, Nakanishi K. Biophys J. 1985;47:509–512. doi: 10.1016/S0006-3495(85)83944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ottolenghi M, Sheves M. J Membr Biol. 1989;112:193–212. doi: 10.1007/BF01870951. [DOI] [PubMed] [Google Scholar]
- 30.Fang J, Cariker J, Balogh-Nair V, Nakanishi K. J Am Chem Soc. 1983;105:5162–5163. [Google Scholar]
- 31.Brock A, Muradin-Szweykowska M, Courtin J, Lugtenburg J. Recl Trav Chim Pays-Bas. 1983;102:46–52. [Google Scholar]
- 32.Sheves M, Friedman N, Albeck A, Ottolenghi M. Biochemistry. 1985;21:1260–1265. [Google Scholar]
- 33.Battacharya S, Marti T, Otto H, Heyn M, Khorana G. J Biol Chem. 1992;267:6757–6762. [PubMed] [Google Scholar]
- 34.Sussman J, Harel M, Frolow F, Oefner C, Goodman A, Toker L, Silman I. Science. 1991;253:872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- 35.Dupuis P, Harris I, Sandorfy C, Leclerq J, Vocelle D. Rev Can Biol. 1980;39:247–258. [PubMed] [Google Scholar]
- 36.Hildebrandt P, Stockburger M. Biochemistry. 1984;23:5539–5548. [Google Scholar]
- 37.De Groot H, Harbison G, Herzfeld J, Griffin R. Biochemistry. 1989;28:3346–3353. doi: 10.1021/bi00434a033. [DOI] [PubMed] [Google Scholar]
- 38.Gat Y, Sheves M. J Am Chem Soc. 1993;115:3772–3773. [Google Scholar]
- 39.Humphrey W, Logunov I, Schulten K, Sheves M. Biochemistry. 1994;33:3668–3678. doi: 10.1021/bi00178a025. [DOI] [PubMed] [Google Scholar]
- 40.Maeda A, Sasaki J, Yumazaki Y, Needleman R, Lanyi J. Biochemistry. 1994;33:1713–1717. doi: 10.1021/bi00173a013. [DOI] [PubMed] [Google Scholar]
- 41.Douhal A. Science. 1997;276:221. [Google Scholar]