Abstract
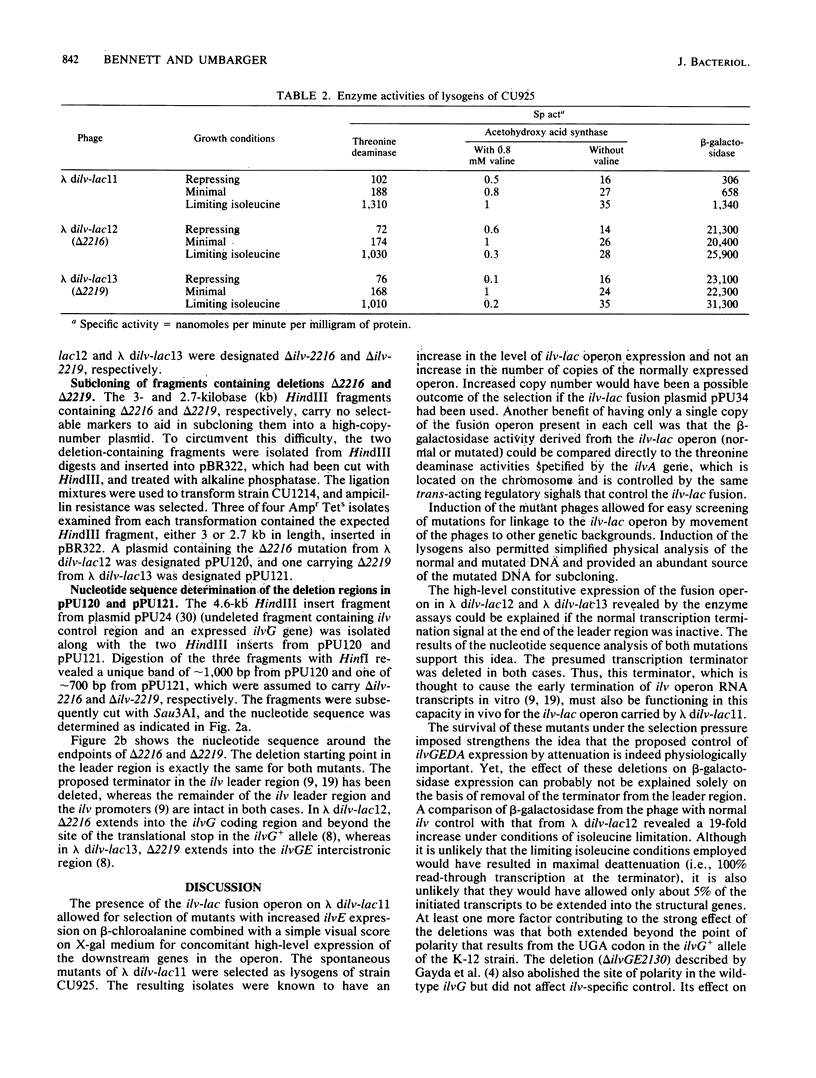
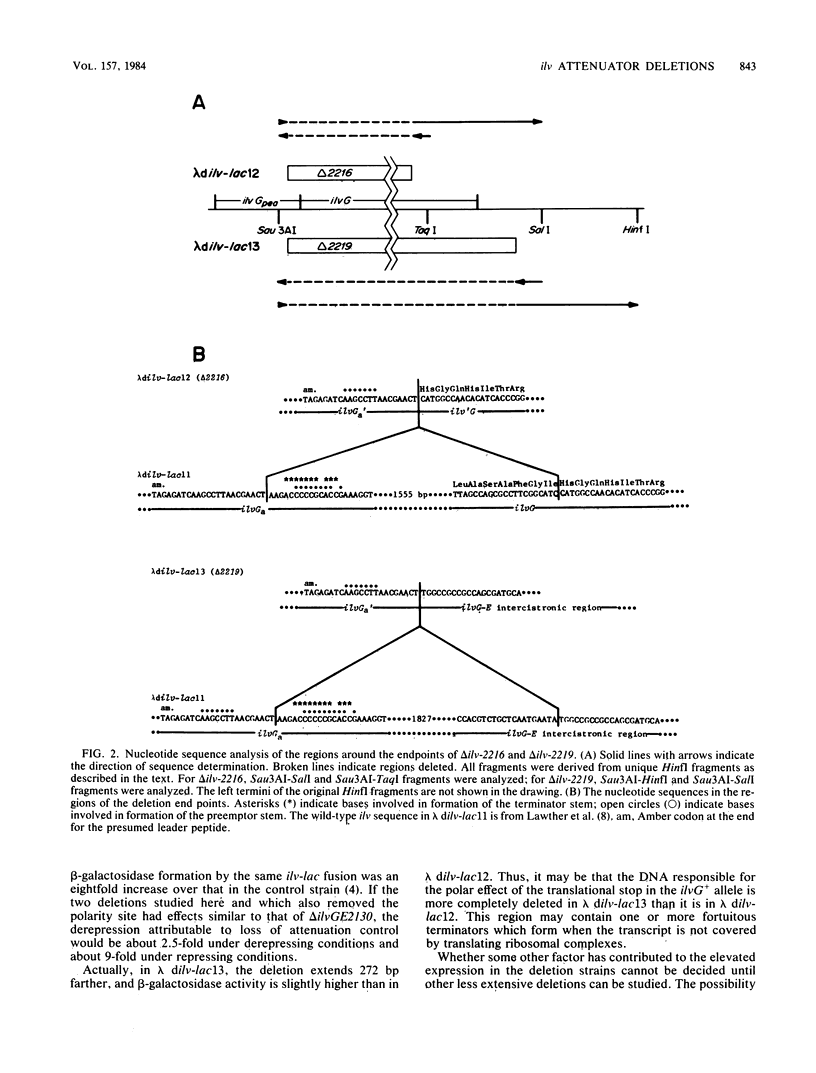
A lysogenizing lambda phage, lambda dilv-lac11, was constructed to carry an ilvD-lac operon fusion. Expression from the phage of the ilvE and lacZ genes is controlled by an intact ilv control region also carried by this phage. Two spontaneous mutants of lambda dilv-lac11 that have high-level constitutive expression of the ilv-lac fusion operon were isolated by growth on a beta-chloroalanine selective medium. The mutants were shown by nucleotide sequence determination to contain large deletions (delta 2216, approximately 1.6 kilobases; delta 2219, approximately 1.9 kilobases), which in both cases remove the proposed ilv attenuator terminator. The rest of the ilv leader and promoter region DNA remains intact in these mutants. Deletion 2216 also removed part of the downstream ilvG gene, whereas delta 2219 extended through the entire ilvG gene into the ilvGE intercistronic region. A possible mechanism of deletion formation is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfin S. M., Koziell D. A. Inhibition of growth of Salmonella typhimurium and of threonine deaminase and transaminase B by beta-chloroalanine. J Bacteriol. 1971 Feb;105(2):519–522. doi: 10.1128/jb.105.2.519-522.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh P. J., Schmeissner U., Hofer M., Miller J. H. Genetic studies of the lac repressor. VII. On the molecular nature of spontaneous hotspots in the lacI gene of Escherichia coli. J Mol Biol. 1978 Dec 25;126(4):847–857. doi: 10.1016/0022-2836(78)90023-2. [DOI] [PubMed] [Google Scholar]
- Favre R., Wiater A., Puppo S., Iaccarino M. Expression of a valine-resistant acetolactate synthase activity mediated by the ilv O and ilv G genes of Escherichia coli K-12. Mol Gen Genet. 1976 Feb 2;143(3):243–252. doi: 10.1007/BF00269400. [DOI] [PubMed] [Google Scholar]
- Gayda D. J., Leathers T. D., Noti J. D., Smith F. J., Smith J. M., Subrahmanyam C. S., Umbarger H. E. Location of the multivalent control site for the ilvEDA operon of Escherichia coli. J Bacteriol. 1980 May;142(2):556–567. doi: 10.1128/jb.142.2.556-567.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D., Saedler H. IS2-61 and IS2-611 arise by illegitimate recombination from IS2-6. Mol Gen Genet. 1979 Oct 3;176(2):233–238. doi: 10.1007/BF00273217. [DOI] [PubMed] [Google Scholar]
- Gray J. E., Bennett D. C., Umbarger H. E., Calhoun D. H. Physical and genetic localization of ilv regulatory sites in lambda ilv bacteriophages. J Bacteriol. 1982 Mar;149(3):1071–1081. doi: 10.1128/jb.149.3.1071-1081.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. I., Somerville R. L. Evidence that repression mechanisms can exert control over the thr, leu, and ilv operons of Escherichia coli K-12. J Bacteriol. 1983 Jul;155(1):49–55. doi: 10.1128/jb.155.1.49-55.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawther R. P., Calhoun D. H., Adams C. W., Hauser C. A., Gray J., Hatfield G. W. Molecular basis of valine resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 Feb;78(2):922–925. doi: 10.1073/pnas.78.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Hatfield G. W. Multivalent translational control of transcription termination at attenuator of ilvGEDA operon of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1862–1866. doi: 10.1073/pnas.77.4.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leathers T. D., Noti J., Umbarger H. E. Physical characterization of ilv-lac fusions. J Bacteriol. 1979 Oct;140(1):251–260. doi: 10.1128/jb.140.1.251-260.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Meagher R. B., Shepherd R. J., Boyer H. W. The structure of cauliflower mosaic virus. I. A restriction endonuclease map of cauliflower mosaic virus DNA. Virology. 1977 Jul 15;80(2):362–375. doi: 10.1016/s0042-6822(77)80012-3. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., Weisberg R., Enquist L., Mizuuchi M., Buraczynska M., Foeller C., Hsu P. L., Ross W., Landy A. Structure and function of the phage lambda att site: size, int-binding sites, and location of the crossover point. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):429–437. doi: 10.1101/sqb.1981.045.01.057. [DOI] [PubMed] [Google Scholar]
- Nargang F. E., Subrahmanyam C. S., Umbarger H. E. Nucleotide sequence of ilvGEDA operon attenuator region of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1823–1827. doi: 10.1073/pnas.77.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. II. IDENTIFICATION OF TWO OPERATOR GENES. J Bacteriol. 1965 Mar;89:654–660. doi: 10.1128/jb.89.3.654-660.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ross W., Landy A. Patterns of lambda Int recognition in the regions of strand exchange. Cell. 1983 May;33(1):261–272. doi: 10.1016/0092-8674(83)90355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W., Shulman M., Landy A. Biochemical analysis of att-defective mutants of the phage lambda site-specific recombination system. J Mol Biol. 1982 Apr 15;156(3):505–522. doi: 10.1016/0022-2836(82)90263-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. II. Mutations induced by bacteriophage lambda in Escherichia coli K12. J Mol Biol. 1973 Oct 25;80(2):297–314. doi: 10.1016/0022-2836(73)90174-5. [DOI] [PubMed] [Google Scholar]
- Smith J. M., Smolin D. E., Umbarger H. E. Polarity and the regulation of the ilv gene cluster in Escherichia coli strain K-12. Mol Gen Genet. 1976 Oct 18;148(2):111–124. doi: 10.1007/BF00268374. [DOI] [PubMed] [Google Scholar]
- Sommer H., Cullum J., Saedler H. IS2-43 and IS2-44: new alleles of the insertion sequence IS2 which have promoter activity. Mol Gen Genet. 1979 Aug;175(1):53–56. doi: 10.1007/BF00267855. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Subrahmanyam C. S., McCorkle G. M., Umbarger H. E. Physical location of the ilvO determinant in Escherichia coli K-12 deoxyribonucleic acid. J Bacteriol. 1980 May;142(2):547–555. doi: 10.1128/jb.142.2.547-555.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subrahmanyam C. S., Noti J. D., Umbarger H. E. Regulation of ilvEDA expression occurs upstream of ilvG in Escherichia coli: additional evidence for an ilvGEDA operon. J Bacteriol. 1980 Oct;144(1):279–290. doi: 10.1128/jb.144.1.279-290.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzan M., Favre R., Gallay E., Caro L. Genetical and structural analysis of a group of lambda ilv and lambda rho transducing phages. Mol Gen Genet. 1981;182(3):462–470. doi: 10.1007/BF00293936. [DOI] [PubMed] [Google Scholar]
- Vonder Haar R. A., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli K-12: detection and measurement of ilv-specific messenger ribonucleic acid. J Bacteriol. 1974 Nov;120(2):687–696. doi: 10.1128/jb.120.2.687-696.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


