Abstract
End-organ damage is common in patients with sickle cell disease (SCD) thereby limiting the use of allogeneic stem cell transplantation (SCT). We report the outcome of two adult SCD patients, one with end stage renal disease (ESRD), who underwent fludarabine-based non-myeloablative SCT from HLA-identical matched siblings. To prevent fludarabine toxicity, the patient with ESRD underwent aggressive dialysis following adjusted fludarabine dosing. Pharmacokinetics of the fludarabine metabolite F-Ara-A was studied on the patient with ESRD and two additional patients with normal renal function. Both patients with SCD achieved full donor erythroid chimerism, have normal blood counts and are on no immunosuppressive medications. With a 20% dose reduction followed by daily dialysis, we achieved fludarabine drug exposure that is nearly identical to that achieved in patients with normal renal function. We conclude that fludarabine-based non-myeloablative allogeneic SCT for adult patients with SCD is feasible, even in the setting of ESRD.
INTRODUCTION
Allogeneic stem cell transplantation (SCT) cures patients with sickle cell disease (SCD), however the risk/benefit considerations are complex(1–3). As patients age, SCD-related comorbidities increase. This magnifies the importance of a non-myeloablative SCT approach that is less likely to result in graft versus host disease (GvHD), while reliable in providing robust donor stem cell engraftment. Published reports of non-myeloablative SCT for SCD have confirmed improved safety, but the majority of such transplants were unsuccessful due to graft failure(4, 5).
The purine analog fludarabine is a critical component of nearly all non-myeloablative SCT preparative regimens. Since up to 60% of the active metabolite 2-fluoro-ara-A (F-Ara-A) is cleared by the kidneys, use in patients with renal insufficiency or renal failure is hazardous. We report the outcome of two adult patients with SCD, one of whom with end stage renal disease, who underwent non-myeloablative allogeneic SCT using a fludarabine-containing preparative regimen. We performed a comparison of the pharmacokinetics of fludarabine in the context of renal failure and normal renal function.
PATIENTS, MATERIALS, METHODS
Patients
The patients, both with homozygous HgbS, signed the informed consent for participation in an IRB approved clinical trial of non-myeloablative allogeneic stem cell transplantation for treatment of patients with sickle cell disease. The protocol is part of the NHLBI-sponsored Comprehensive Sickle Cell Centers program. Patient #1 is a 21 year-old male with a history of frequent painful episodes and patient #2 is a 27 year-old with dialysis-dependent end-stage renal disease following failure of a kidney graft from his matched sibling, as well as red cell aplasia and transfusion-associated iron overload. Fludarabine pharmacokinetics assessment was performed in patient #2, and in two female patients with hematologic malignancies and normal renal function (creatinine clearance calculated by Cockcroft-Gault formula 65ml/min and 71ml/min, respectively).
Treatment plan
The patients underwent pre-transplant exchange transfusion to achieve HgbA >50%. Conditioning consisted of 200 cGy total body irradiation followed by fludarabine (Pt #1: 30 mg/m2, Pt #2: 24 mg/m2) and cyclophosphamide 500 mg/m2, both given daily for four days, and alemtuzumab 100 mg given in divided doses over 5 days. The fludarabine dose of 24 mg/m2 represents a dose reduction of 20%, which was based on the limited available published experience with fludarabine in the setting of renal failure(6). Mycophenolate mofetil 2 grams/day was given for 100 days following transplantation. Patient #1 and #2 each received a G-CSF mobilized peripheral blood stem cell graft from an HLA-identical sibling containing 21×106 and 19×106 CD34+ cells/kg, respectively. The donor for patient #2 demonstrated 43% HgbS consistent with SCD trait. The graft was incubated with alemtuzumab, 10 mg/150cc ‘in the bag’ for 30 minutes at 37°C prior to infusion. Patient #2 underwent 6 hours of conventional hemodialysis using an F200 dialyzer, 12 hours after each fludarabine dose. Donor chimerism was determined by quantitative PCR amplification of informative microsatellites using DNA isolated from peripheral blood CD3+ and CD15+ cells. Erythroid chimerism was determined by hemoglobin electrophoresis.
Pharmacokinetic studies
Plasma samples from patient #2 and two additional non-SCD patients with normal renal function undergoing stem cell transplantation using the same chemotherapy preparative regimen (but without total body irradiation) were collected over 24 hours after doses 1 and 4 of fludarabine. F-Ara-A was measured by a LC/MS/MS assay that was developed and validated at the Duke Pharmacokinetics laboratory (data not published).
Lymphocyte subset analysis
Lymphocyte subsets (T-cell and natural killer [NK] cells) were enumerated using 2-color multiparameter flow cytometry. Peripheral blood mononuclear cells (PBMCs) were prepared by Ficoll separation of freshly drawn, heparinized whole blood. Samples were cryopreserved at −70°C and processed in bulk. Cells were stained with fluorescence-labeled monoclonal antibodies with specificity to anti-CD2, -CD3, -CD4, -CD8, -CD16, and -CD56. Analysis was performed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Results and discussion
Treatment-related toxicity
Both patients tolerated the conditioning regimen without unanticipated complications. Patient #1 never required hospital admission. Patient #2 was hospitalized for administration of the conditioning regimen in order to facilitate intensive dialysis. The peri-transplant hematologic parameters are demonstrated in Figures 1A,C. Patient #1 and #2 were neutropenic (ANC<500) for 3 and 12 days, respectively. Patient #1 platelet nadir was 141,000. Patient #2 required 6 platelet transfusions to maintain the protocol-mandated minimum count of 50,000.
Figure 1. Hematopoietic recovery and donor/recipient chimerism following transplantation.
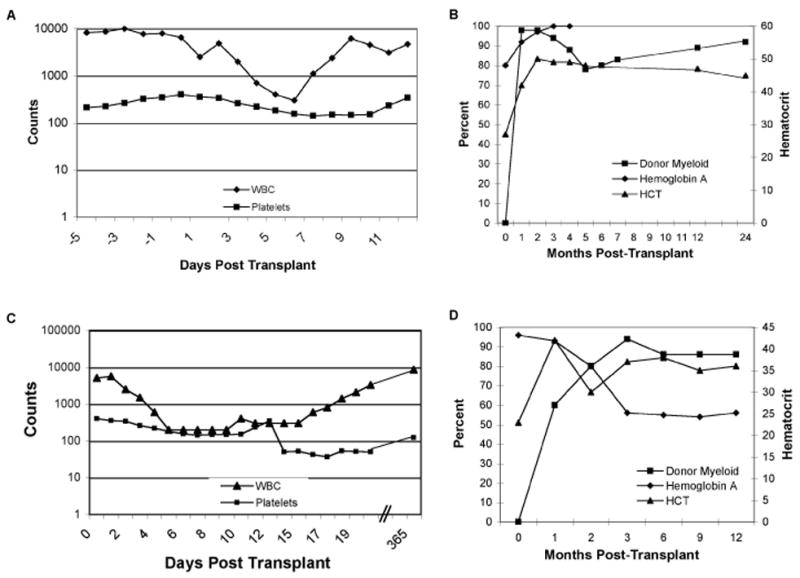
(A) Sickle cell disease patient #1 white blood cell count and platelet count during the peri-transplant period. (B) Sickle cell disease patient #1 donor/recipient myeloid and erythroid (as measured by percent hemoglobin A) chimerism and hematocrit following stem cell transplantation. (C) Sickle cell disease patient #2 white blood cell count and platelet count during the peri-transplant period. (D) Sickle cell disease patient #2 donor/recipient myeloid and erythroid (as measured by percent hemoglobin A) chimerism and hematocrit following stem cell transplantation. Of note, the donor for patient #2 has sickle cell trait. Elevated pre-transplantation hemoglobin A reflects protocol-mandated exchange transfusions.
Neither patient had adverse drug effects attributable to fludarabine toxicity. Neither patient experienced acute GvHD, and with a median follow-up of 20 months, neither has experienced chronic GvHD. However, four months post transplant, patient #2 developed symptomatic heart failure. His echocardiogram, which was normal prior to transplantation, demonstrated moderate left ventricular dilatation, a small pericardial effusion and an ejection fraction of 35%. Although the ejection fraction dropped to a low of 20%, a cardiac MUGA scan performed 15 months post-transplant revealed significant recovery to 38%.
Hematopoietic recovery, immune recovery and engraftment
Myeloid and erythroid chimerism, along with hematocrit are demonstrated in Figures 1B,D. Both patients have stable donor T-cell (CD3+) chimerism. At 18 months of follow-up, patient #1 has 85% donor T-cells and at 12 months of follow-up, patient #2 has 58% donor T-cells. By 3 months following transplantation, both patients achieved full donor erythroid chimerism as demonstrated by a hemoglobin electrophoresis pattern that mirrored that of their stem cell donor. This resulted in normalization of the hematocrit levels. Patient #2 began therapeutic phlebotomy at 90 days post-transplant to address iron overload. At 1 year post transplant, both patients had CD4 and CD8 counts approaching normal limits(7) (CD3 525 and 554; CD4 292 and 325 cells per μl, patient 1 and 2, respectively). At 2 years of follow-up, patient #1 had normal numbers of T-cells and B-cell (data not shown). Interestingly, absolute numbers of NK cells were persistently low in both patients for the duration of the follow-up period.
F-ara-A pharmacokinetics
F-ara-A pharmacokinetics in our SCD patient with renal failure was compared to two patients with normal renal function (Figure 3). With a 20% fludarabine dose reduction and once daily intensive dialysis, we achieved nearly identical drug exposure (AUC 4093 mg·h/L vs mean 4010 mg·h/L). As a consequence of the 20% dose reduction, the maximum concentration of F-ara-A was lower in the renal failure patient, but this was compensated for by lower drug clearance (5.3 l/h vs mean 6.4 l/h).
Figure 3.
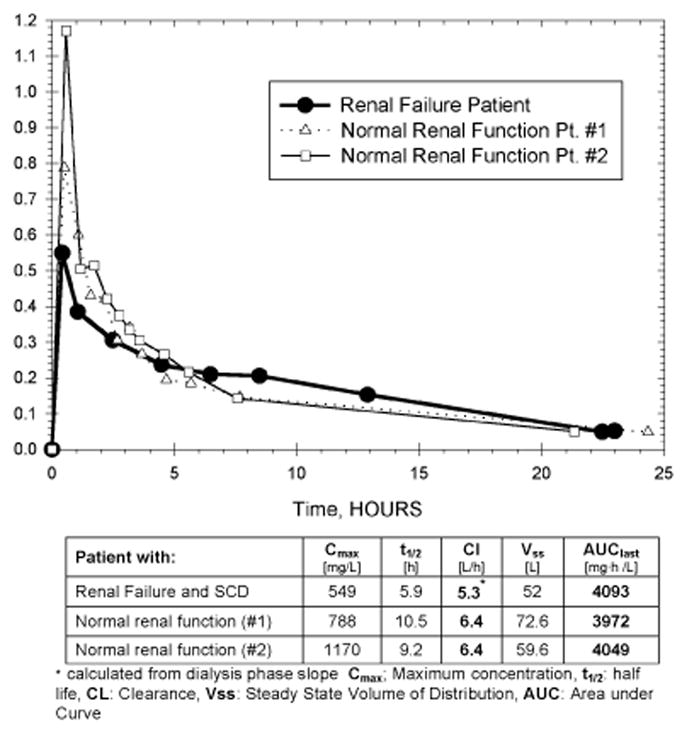
F-ara-A pharmacokinetics in a patient with dialysis-dependent renal failure and sickle cell disease and two patients with normal renal function.
Discussion
Recently described clinical and laboratory characteristics such as pulmonary hypertension, renal insufficiency and leukocytosis along with the intensity of hemolysis allow for the identification of SCD patients who are destined to succumb to complications before the median survival of approximately 40–50 years(8–10). These are the patients that should be targeted for a safe and reliable allogeneic stem cell transplant approach. The ideal approach would be one that combines a suitably matched sibling or possibly unrelated donor with a non-myeloablative preparative regimen that incorporates T-cell depletion to protect against graft versus host disease. Of the 15 SCD patients reported in the literature that have undergone non-myeloablative SCT, only 6 have engrafted(4, 5, 11–14). Of these, 3 developed acute or chronic GvHD. We report 2 consecutive adult patients who were successfully transplanted with an alemtuzumab-containing non-myeloablative regimen that has been shown to significantly reduce the risk of GvHD(15–17). Despite the use of Alemtuzumab, which is known to compromise post-transplant immune recovery, we observed robust immune recovery in both transplant recipients. This is attributable to their relatively young age, lack of pre-transplant chemotherapy exposure, and the absence of GvHD.
There is limited published experience with the use of fludarabine in the setting of renal impairment(6). Severe neurotoxicity is of primary concern in this patient population given the potential for excessively high levels of F-ara-A(18). The approach employed in our case was based on that of Kielstein et al who reported F-ara-A pharmacokinetic data in an anuric patient given fludarabine for treatment of chronic lymphocytic leukemia(19). Using a 20% dose reduction of fludarabine and intensive daily dialysis, this regimen can be applied to patients with end stage renal disease. While it is likely that cyclophosphamide toxicity is the primary cause of the patient’s cardiac pathology(20), it is possible that cardiac iron deposition (pre-transplant ferritin, 7200 ng/ml) was a contributing factor.
The more severe bone marrow suppression observed in the patient with renal failure suggests the possible need for cyclophosphamide dose reduction in such patients. However further experience is needed for this observation to be confirmed.
Figure 2. T-cell and Natural Killer cell recovery following stem cell transplantation.
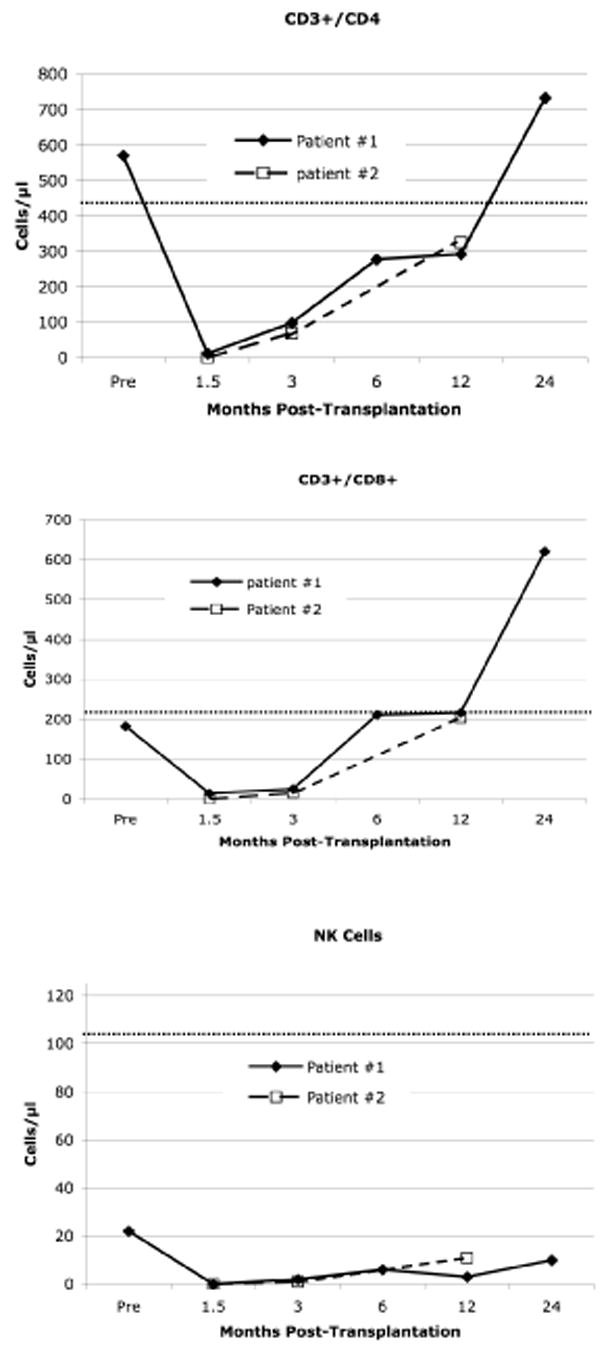
Pre-transplant and 6-month post-transplant data points are not available for patient #2
Acknowledgments
The study investigators wish to thank the nurse practioners, physician’s assistants, ward and clinic nurses and staff of the Duke Adult Stem Cell Transplant Program for their outstanding care of the patients described in this report. This work was supported by the NHLBI-sponsored Comprehensive Sickle Cell Centers Program (U54 HL070769-02) (D.R. and M.T.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walters MC, Patience M, Leisenring W, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335:369–376. doi: 10.1056/NEJM199608083350601. [see comments] [DOI] [PubMed] [Google Scholar]
- 2.Panepinto JA, Walters MC, Carreras J, et al. Matched-related donor transplantation for sickle cell disease: report from the Center for International Blood and Transplant Research. Br J Haematol. 2007;137:479–485. doi: 10.1111/j.1365-2141.2007.06592.x. [DOI] [PubMed] [Google Scholar]
- 3.Walters MC, Storb R, Patience M, et al. Impact of bone marrow transplantation for symptomatic sickle cell disease: an interim report. Multicenter investigation of bone marrow transplantation for sickle cell disease. Blood. 2000;95:1918–1924. [PubMed] [Google Scholar]
- 4.Iannone R, Casella JF, Fuchs EJ, et al. Results of minimally toxic nonmyeloablative transplantation in patients with sickle cell anemia and beta-thalassemia. Biol Blood Marrow Transplant. 2003;9:519–528. doi: 10.1016/s1083-8791(03)00192-7. [DOI] [PubMed] [Google Scholar]
- 5.Horan JT, Liesveld JL, Fenton P, Blumberg N, Walters MC. Hematopoietic stem cell transplantation for multiply transfused patients with sickle cell disease and thalassemia after low-dose total body irradiation, fludarabine, and rabbit anti-thymocyte globulin. Bone Marrow Transplant. 2005;35:171–177. doi: 10.1038/sj.bmt.1704745. [DOI] [PubMed] [Google Scholar]
- 6.Lichtman SM, Etcubanas E, Budman DR, et al. The pharmacokinetics and pharmacodynamics of fludarabine phosphate in patients with renal impairment: a prospective dose adjustment study. Cancer investigation. 2002;20:904–913. doi: 10.1081/cnv-120005903. [DOI] [PubMed] [Google Scholar]
- 7.Storek J, Dawson MA, Maloney DG. Normal T, B, and NK cell counts in healthy donors at 1 year after blood stem cell harvesting. Blood. 2000;95:2993–2994. [PubMed] [Google Scholar]
- 8.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 9.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 10.Sebastiani P, Nolan V, Clinton B, et al. A network model to predict the risk of death in sickle cell disease. Blood. 2007 doi: 10.1182/blood-2007-04-084921. ePub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Besien K, Bartholomew A, Stock W, et al. Fludarabine-based conditioning for allogeneic transplantation in adults with sickle cell disease. Bone Marrow Transplant. 2000;26:445–449. doi: 10.1038/sj.bmt.1702518. [DOI] [PubMed] [Google Scholar]
- 12.van Besien K, Stock W, Smith S, et al. Allogeneic stem cell transplantation with fludarabine, melphalan, and campath conditioning for adults with advanced sickle cell disease. Blood. 2002;100:430b. [Google Scholar]
- 13.Krishnamurti L, Blazar BR, Wagner JE. Bone marrow transplantation without myeloablation for sickle cell disease. N Engl J Med. 2001;344:68. doi: 10.1056/NEJM200101043440119. [DOI] [PubMed] [Google Scholar]
- 14.Schleuning M, Stoetzer O, Waterhouse C, Schlemmer M, Ledderose G, Kolb HJ. Hematopoietic stem cell transplantation after reduced-intensity conditioning as treatment of sickle cell disease. Exp Hematol. 2002;30:7–10. doi: 10.1016/s0301-472x(01)00775-5. [DOI] [PubMed] [Google Scholar]
- 15.Kottaridis PD, Milligan DW, Chopra R, et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood. 2000;96:2419–2425. [PubMed] [Google Scholar]
- 16.Rizzieri DA, Koh LP, Long GD, et al. Partially matched, nonmyeloablative allogeneic transplantation: clinical outcomes and immune reconstitution. J Clin Oncol. 2007;25:690–697. doi: 10.1200/JCO.2006.07.0953. [DOI] [PubMed] [Google Scholar]
- 17.Tauro S, Craddock C, Peggs K, et al. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J Clin Oncol. 2005;23:9387–9393. doi: 10.1200/JCO.2005.02.0057. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Vena DA, Foss FM, Sorensen JM. Neurotoxicity of purine analogs: a review. J Clin Oncol. 1994;12:2216–2228. doi: 10.1200/JCO.1994.12.10.2216. [DOI] [PubMed] [Google Scholar]
- 19.Kielstein JT, Stadler M, Czock D, Keller F, Hertenstein B, Radermacher J. Dialysate concentration and pharmacokinetics of 2F-Ara-A in a patient with acute renal failure. European journal of haematology. 2005;74:533–534. doi: 10.1111/j.1600-0609.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- 20.Cazin B, Gorin NC, Laporte JP, et al. Cardiac complications after bone marrow transplantation. A report on a series of 63 consecutive transplantations. Cancer. 1986;57:2061–2069. doi: 10.1002/1097-0142(19860515)57:10<2061::aid-cncr2820571031>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]


