Abstract
The signaling pathway of cyclooxygenase-2 (COX-2) induction following ischemic preconditioning (IPC) in brain remains undefined. To determine role of COX-2 in ischemic preconditioning, we used two in vitro models: mixed cortical neuron/astrocyte cell cultures and organotypic hippocampal slice cultures. We simulated IPC by exposing cell or slice cultures to 1 h or 15 min of oxygen/glucose deprivation (OGD), respectively, 48 h prior to ischemia. To mimic ischemia in vitro, we exposed cell or slice cultures to OGD of 4 h or 45 min, respectively. In cell cultures, these experiments revealed that COX-2 induction peaked at 24 h following IPC in cell culture. Inhibition of COX-2 activation with 50 μM NS-398 (a COX-2 selective inhibitor) abolished IPC-mediated neuroprotection in both in vitro models. Next, we tested whether εPKC and ERK1/2 activation were involved in IPC-mediated neuroprotection and COX-2 expression in cell culture. Cell cultures were treated with an εPKC-specific activating peptide (ψεRACK, 100 nM) for 1 h, and 48 h later were exposed to OGD. εPKC activation increased ERK1/2 phosphorylation and COX-2 induction and conferred neuroprotection similar to IPC. Additionally, inhibition of either εPKC or ERK1/2 activation abolished COX-2 expression and neuroprotection due to ischemic preconditioning. These results demonstrate a crucial role for the εPKC → ERK1/2 → COX-2 pathway in the induction of neuroprotection via ischemic preconditioning.
Keywords: Neuroprotection, ERK1/2, mixed cortical neuron/astrocyte cell cultures, organotypic hippocampal slice cultures, ischemia, oxygen/glucose deprivation
INTRODUCTION
Ischemic preconditioning (IPC) is an endogenous protective mechanism invoked by a brief, sublethal ischemic insult. Ischemic preconditioning can reduce cell injury caused by a subsequent lethal ischemic insult. Many proteins exhibit increased expression after IPC, including aldose reductase (Shinmura et al., 2002a), and antioxidant enzymes such as MnSOD (Hoshida et al., 1993), nitric oxide synthase (NOS) (Gonzalez-Zulueta et al., 2000), and cyclooxygenase (COX) in both heart and brain (Shinmura et al., 2000, Gendron et al., 2005). In the present study we focused on the role of COX after IPC.
Cyclooxygenase-2 (COX-2) is the rate-limiting enzyme for prostaglandin (PG) synthase, catalyzing the conversion of arachidonic acid to prostaglandin H2 (PGH2) (Smith et al., 1996). COX has two distinct isoforms: (1) COX-1, which is constitutively expressed for prostanoid formation and is present in most cells (Bazan et al., 1994); (2) COX-2, which is rapidly induced by cytokines and various stress stimuli but is also constitutively expressed in brain, heart and kidney (Yasojima et al., 1999). Inhibitors of COX-2 reduce brain injury in response to global ischemia and excitotoxicity (Nakayama et al., 1998, Kim et al., 2001). COX-2-deficient mice had reduced hippocampal neuronal cell injury after transient forebrain ischemia (Sasaki et al., 2004). Inhibition of COX-2 activity reduced cardiac damage after myocardial infarction (LaPointe et al., 2004).Thus, COX-2 activity contributes to ischemia/reperfusion injury in brain and heart.
In direct contrast, COX-2 induction has also been implicated as a mediator of cell protection (McAdam et al., 1999). For example, a) COX-2 activation protects cardiomyocytes against oxidative stress (Adderley and Fitzgerald, 1999); b) Inhibition of COX-2 exacerbates the inflammation and hippocampal neuronal death induced by seizures (Baik et al., 1999, Gilroy et al., 1999); c) Prostaglandin E2 (PGE2), the product of COX-2 activity, leads to neuroprotection in cerebral ischemia (McCullough et al., 2004). Furthermore, these protective roles of COX-2 activity were also observed in ischemic preconditioning. Shinmura et al. showed that COX-2 expression was upregulated after ischemic preconditioning (Shinmura et al., 2000), and inhibition of COX-2 activity after ischemic preconditioning abolished the increase of PG production and cardioprotective effects of preconditioning in heart. In cortical neurons, the COX-2 inhibitor SC-58125 abolished ischemic preconditioning, and COX-2-induced PGE2 showed neuroprotection against oxygen/glucose deprivation (Gendron et al., 2005). Recent experiments have demonstrated that cortical spreading depression induces COX-2 expression and confers neuroprotection against ischemia (Horiguchi et al., 2006). A major gap in our understanding remains the signaling pathways that induce COX-2 expression following ischemic preconditioning.
Signaling pathways which generate the mediators of IPC include εPKC (Raval et al., 2003, Lange-Asschenfeldt et al., 2004), protein tyrosine kinase (PTK-Src/LcK) (Ludwig et al., 2004), mitogen-activated protein kinase (MAPK-p38/ERK1/2) (Blanco et al., 1995), and nuclear factor - kappa B (NF-κB) (von Knethen et al., 1999). All of these signaling pathways are known to regulate COX-2 expression in heart and brain (von Knethen et al., 1999, Allport et al., 2000, Shinmura et al., 2002b). εPKC and p38 MAPK activation mediated COX-2 expression induced by oxygen/glucose deprivation in cardiac fibroblasts (Takeda et al., 2004). Previous studies from our laboratory demonstrated that a protein kinase C isozyme, namely εPKC, plays an essential role in the neuroprotective effects of ischemic preconditioning in the organotypic hippocampal slice. Therefore, in this study, we tested the hypothesis that ischemic preconditioning induces COX-2 via εPKC and ERK1/2 signaling pathways.
EXPERIMENTAL PROCEDURES
All animal procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health and were approved by the Animal Care and Use Committee of the University of Miami. According to these guidelines, efforts were made to minimize the number of animals and their discomfort.
Preparation of mixed cortical neuron/astrocyte cell cultures (cell cultures)
Sprague-Dawley neonatal (1-2 days old) rats were anesthetized by intraperitoneal injection of ketamine (1.0 mg/pup). Animals were decapitated and the brains quickly removed. For mixed cortical neuron/astrocyte cell cultures, at first, astrocytes were prepared from neonatal rat as described previously (Kim et al., 2002a). Dissociated astrocytes were plated with minimum essential medium (MEM) (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS), 10% equine serum, 2 mM glutamine and 1 μg/ml epidermal growth factor (Sigma, St. Louis, MO,) in three cortical hemisphere/24-well plates. After 10 days cortical neurons were plated on top of astrocytes to generate co-culture. To isolate cortical neurons, nineteen days pregnant Sprague-Dawley rats were anesthetized by halothane and embryos were quickly removed and decapitated. The cerebral cortices of the embryos were isolated and dissociated cortical neurons were cultured on a confluent monolayer of astrocytes with MEM containing 5% FBS, 5% equine serum and 2 mM glutamine. Cells were used after 10-11 days in vitro.
Preparation of organotypic hippocampal slice cultures (slice cultures)
Organotypic hippocampal slice cultures were prepared as described previously (Xu et al., 2002, Raval et al., 2003). In brief, neonatal (9 to 11 days old) Sprague–Dawley rats were anesthetized by intraperitoneal injection of ketamine (1.0 mg/pup). Animals were decapitated and the brains quickly removed. Transverse slices (400 μm) were dissected from the hippocampi and placed in Gey's Balanced Salt Solution (Sigma, St. Louis, MO) supplemented with 6.5 mg/mL glucose at 4°C. After one hour, 2 slices were placed onto one 30 mm diameter membrane insert (Millicell-CM, Millipore, Bedford, MA) and inserts were transferred to 6-well culture plates with 1 ml of culture medium per well. Culture medium consisted of 50% Minimum Essential Medium, 25% Hank's Balanced Salt Solution (HBSS), 25% horse serum (Sigma, St. Louis, MO) supplemented with 6.5 mg/mL glucose and 1 mM glutamine. Slice cultures were incubated (equilibrated at 36°C, 5% CO2, humidity 100%) for 14-15 days before experiments were performed.
Scheme of oxygen/glucose deprivation (OGD) injury
To mimic ischemic injury, we exposed cells/slices to oxygen/glucose deprivation of 4 h/45 min, respectively. To simulate ischemic preconditioning, cell cultures and slice cultures were exposed to OGD for a short period of 1 h or 15 min, respectively, 48 h prior to ischemia. For OGD, cell cultures and slice cultures were washed twice with glucose-free HBSS (pH 7.4) of the following constitution: 1.26 mM CaCl2·2H2O, 5.37 mM KCl, 0.44 mM KH2PO4, 0.49 mM MgCl2, 0.41 mM MgSO4· 7H2O, 136.9 mM NaCl2, 4.17 mM NaHCO3, 0.34 mM Na2HPO4 .7H2O, 15 mM sucrose (Sigma, St. Louis, MO). The cell cultures and slice cultures were then transferred into an anaerobic chamber (PROOX model 110, BioSpherix, Ltd. Redfield, NY) which was placed in a water-jacketed incubator gassed with 95% N2 / 5% CO2 at 37°C. Then, the chamber was sealed and incubated for 1 h or 15 min (preconditioning) or 4 h or 45 min (ischemic insult) for the cell cultures and slice cultures respectively. Following OGD, the cell cultures and slice cultures were transferred to their respective normal culture media and placed back into the incubator.
Assessment of cell death of mixed cortical neuron/astrocyte cell cultures and organotypic hippocampal slice cultures
To determine assessment of cell death of cell cultures, celluar cytotoxicity was measured by lactate dehydrogenase (LDH) released for 48 h into culture medium (Koh and Choi, 1987). Maximal neuronal LDH release was measured in the neuronal cultures exposed to NMDA (500 μM; 24 h; maximal neuronal death). LDH release was measured at absorbance at 340 nm using a microplate reader (Molecular Devices, Sunnyvale, CA). Values were expressed relative to LDH measurement from maximal neuronal LDH.
To determine the extent of neuronal damage in organotypic slice cultures, we used the propidium iodide (PI) method (Xu et al., 2002, Raval et al., 2003). Slices were incubated in culture media supplemented with 2 μg/mL PI (Sigma, St. Louis, MO) for 1 h. Images were taken using an inverted fluorescence microscope (Olympus IX 50), equipped with a light-intensifying SPOT charge-device-based (CCD) camera (Diagnostic Instruments Inc., Sterling Heights, MI) and SPOT Advanced software. Images of the cultured slices were taken (1) as baseline prior to the preconditioning; (2) 24 h after the preconditioning; (3) 24 h after the ‘test’ ischemic insult; and (4) 24 h after NMDA treatment in order to assess maximal neuronal death. The hippocampal CA1 subfield was chosen as region of interest, and quantification was performed using Scion Image software. The percentage of relative optical intensity (ROI) served as an index of neuronal cell death. Relative cell death was calculated from each ROI as follows: Relative % cell death = (Fexp − Fmin)/ (Fmax − Fmin) ×100, where Fexp is the fluorescence of the test condition, Fmax is maximum fluorescence (100 μM NMDA treatment for 1 h), and Fmin is background fluorescence (prior to preconditioning or OGD). In all groups, experiments were terminated by superfusing slices with an overdose of NMDA (100 μM) 24 h after the end of the experiments to determine the total number of neurons using the PI technique (Fmax above).
Western blotting
Cells were lysed in a lysis buffer containing 1% Nonidet P-40, 20 mM Tris (pH 8.0) 137 mM NaCl, 0.5 mM EDTA, 10% glycerol, 10 mM sodium pyrophosphate, 10 mM sodium fluoride, 1 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM vanadate, and 1 mM phenylmethylsulfonyl fluoride (Raval et al., 2003). Equal amounts of proteins were subjected to 8-12% SDS-PAGE and the separated proteins were electrophoretically transferred to polyvinylidene difluoride membranes. The blot was blocked with 5% non-fat dried milk, incubated overnight at 4°C with anti-COX-2 antibody (1:1,000, Cayman Chemicals, Ann Arbor, MI), β-actin (1:5,000, Sigma St. Louis, MO), pERK1/2 and ERK1/2 (1:1,000, Cell Signaling Technology, Danvers, MA). Then, incubation was followed by horseradish peroxidase-conjugated-specific secondary antibody for 1 h at room temperature. The immunoreactive bands were revealed by ECL western blotting detection reagents (Amersham, Buckinghamshire, England). Western blot images were digitized at eight-bit precision by means of a CCD camera (eight to 12 bit, Xillix Technologies Corporation, Vancouver, Canada) equipped with a 55 mm Micro-Nikkor lens (Nikon, Japan). The camera was interfaced to an advanced image-analysis system (MCID Model M2, Imaging Research, Inc., St. Catherines, Ontario, Canada). The digitized immunoblots were subjected to densitometric analysis using MCID software.
Immunocytochemistry
Mixed cortical neuron/ astrocyte cell cultures were grown onto cover glass in 24-well plates and cultured in vitro until days 10-11 as described above. At 24 h after IPC, cells were fixed with 4% paraformaldehyde in phosphate buffer (10 mM pH 7.4). After washing with PBS-T (0.4% triton X-100 in phosphate buffered saline (PBS)), cells were blocked in 10% goat serum, 10% horse serum and 10% bovine serum albumin (BSA) in PBS-T and incubated with primary antibody against COX-2 (1:1,000, Cayman Chemicals, Ann Arbor, MI), NeuN (Neuron nuclear, a neuronal specific marker) and GFAP (Glial fibrillary acidic protein, an astrocytes marker) (1:100, and 1:400 Chemican, Temeculal, CA) in 5% goat serum, 5% horse serum and 10% BSA in PBS-T. Incubations were performed for 24 h at 4°C. After several washes in PBS-T, cells were incubated with biotinylated anti-rabbit and rhodamine- labeled anti-mouse secondary antibodies (1:500, Vector, Burlingame, CA) for 1 h at 37°C. After several washes in PBS-T, cells were incubated with avidin-conjugated FITC (1:500, Sigma) for 30 min at 37°C. Cells were mounted on slides using Prolong Antifade Kit (Molecular Probes, OR) and were visualized by Laser Scanning Microscopy (LSM) (Carl Zeiss Inc., Germany). The images of cells were analyzed using an LSM 5 image browser.
Statistical Analysis
Data were expressed as the mean± S.E.M. An analysis of variance (ANOVA) followed by Dunnett's multiple comparison tests was used for statistical comparison. In all cases, a p value less than 0.05 was considered statistically significant.
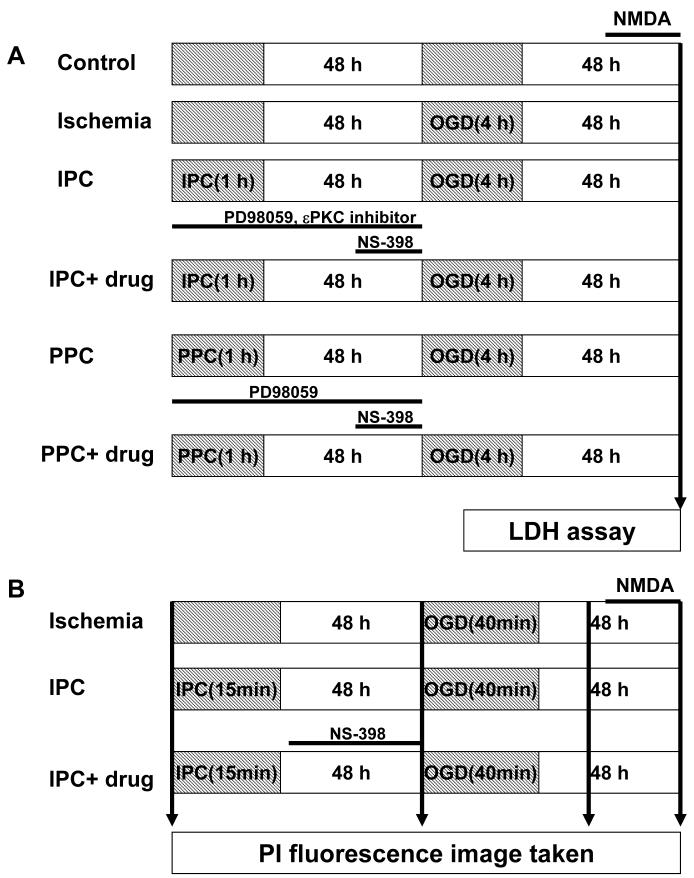
Experimental design
The mixed cortical neuron/astrocyte cell cultures were divided six major groups, as follows (Fig.1A).
Fig.1.
Description of different experimental designs. (A) Mixed cortical neuron/astrocyte cell cultures (B) Organotypic hippocampal slice cultures.
Control: after 10 days in vitro, cell death was measured by the 48 h LDH assay.
Ischemia: cell cultures were exposed to sham ischemic preconditioning, and 48 h later, 4 h of OGD was induced. Immediately following OGD, cell death was measured by the 48 h LDH assay.
Ischemic preconditioning (IPC): cell cultures were exposed to ischemic preconditioning (1 h of OGD), and 48 h of reperfusion later, 4 h of OGD was induced followed by the 48 h LDH assay.
IPC+ drug treatment: cell cultures were treated by PD98059 (a MAPK-K inhibitor,10 μM, Sigma, St. Louis, MO) or εPKC specific inhibitory peptide (εV1-1, 2 nM or 20 nM, KAI Pharmaceuticals Inc., 270 Littlefield Ave South San Francisco, CA 94080) during 1 h of IPC and 48 h of reperfusion. NS-398 (a COX-2 inhibitor, 50 μM, Cayman Chemicals, Ann Arbor, MI) was administrated to cell cultures for 24 h of reperfusion just prior to OGD. Following OGD, cell death was measured by the 48 h LDH assay.
Pharmacological preconditioning (PPC): the εPKC-specific activating peptide (ψεRACK 100 nM, KAI Pharmaceuticals Inc., 270 Littlefield Ave South San Francisco, CA 94080) was administered to cell cultures for 1 h. Then, ψεRACK treated medium was replaced with normal media for 48 h. Cell cultures were then exposed to OGD followed by the 48 h LDH assay.
PPC+ drug treatment: the εPKC-specific activating peptide (ψεRACK 100 nM) was administered to cell cultures for 1 h. ψεRACK treated medium was replaced by normal media for 48 h. PD98059 was administrated to cell cultures during PPC and 48 h of reperfusion. NS-398 was administrated to cell cultures for 24 h of reperfusion just prior to OGD. Following OGD, cell death was measure by the 48 h LDH assay.
The slice cultures were divided into three major groups, as follows (Fig.1B):
Ischemia: Slices were exposed to sham IPC, and 48 h later, 45 min of OGD was applied. After 24 h, cell death was measured by PI staining.
IPC: Slices were exposed to IPC (15 min of OGD), and after 48 h of reperfusion, 45 min of OGD was applied. After 24 h, cell death was measured by PI staining.
IPC+ drug treatment: Slices were exposed to IPC (15 min of OGD) and treated with NS-398 for 48 h during reperfusion. This was followed by 45 min of OGD. After 24 h, cell death was measured by PI staining.
RESULTS
Ischemic preconditioning induces COX-2 expression in cortical neuron culture
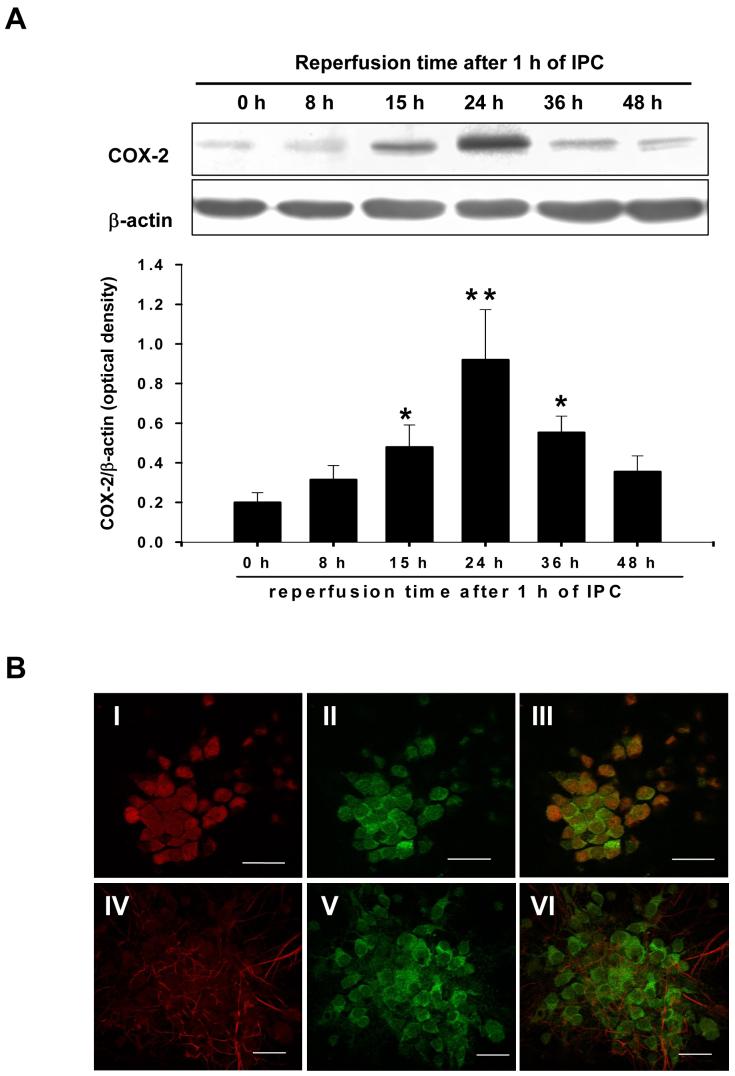
We examined the level of COX-2 expression following the induction of ischemic preconditioning. Mixed cortical neuron/astrocyte cell cultures were preconditioned by exposing cultures to 1 h oxygen/glucose deprivation. COX-2 protein expression was monitored at 8 h, 15 h, 24 h, 36 h and 48 h of reperfusion. This experiment revealed that COX-2 expression was increased at 15 h, peaked at 24 h and persisted at 36 h following IPC (Fig. 2A). Further, to identify the cell type in which COX-2 is being expressed following IPC, we carried out immunocytochemical studies. We found that COX-2 was expressed in neurons, but not in astrocytes at 24 h of reperfusion after IPC (Fig. 2B).
Fig.2.
IPC induced COX-2 protein expression in cortical neurons. (A) Representative western blots of COX-2 and β-actin at 8, 15, 24, 36, and 48 h of reperfusion after 1 h of IPC in mixed cortical neuron/astrocyte cell cultures. Histogram depicting densitometric analysis of immunoblots of COX-2 at various intervals after IPC. Data are the mean ± S.E.M. of normalized densitometry measurements from western blots of COX-2 compared with β-actin (n=8). **p<0.01 compared with 0 h; *p<0.05 compared with 0 h. (B) Confocal microscopic images of mixed cortical neuronal/astrocyte cultures depicting co-localization of immunoreactivities for neuronal specific antibody NeuN (Red) or astrocytes specific antibody GFAP (Red) and COX-2 (Green), at 24 h after IPC. (I,IV) The neurons/astrocytes show positive immunoreactivity for NeuN/GFAP (Red), (II,V) COX-2 immunostaining (Green) and (III,VI) colocalization of NeuN/COX-2 and GFAP/COX-2. Magnification: 40 X; scale bar =20 μM.
COX-2 inhibition prevents neuroprotection in cell cultures and slice cultures
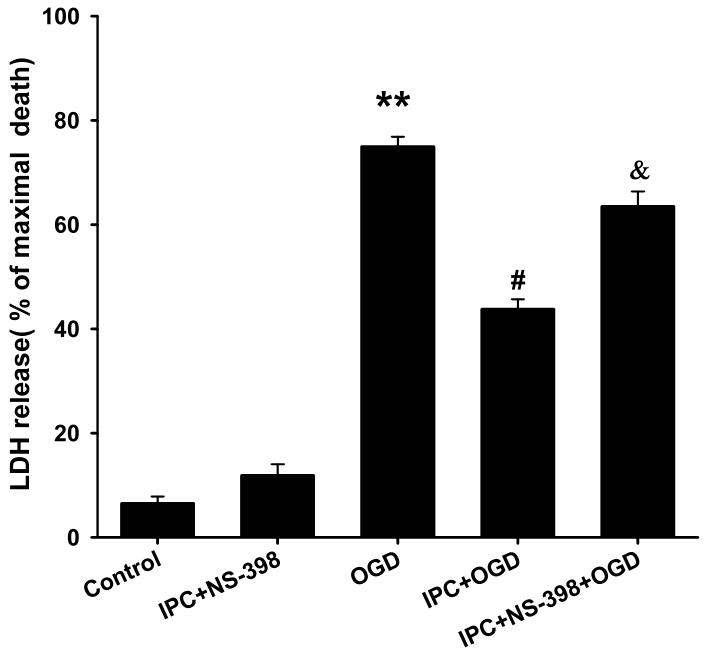
Because we found increased COX-2 expression in cortical neurons following ischemic preconditioning, we asked whether COX-2 activity plays an important role in neuroprotection. To assess cell death in cell cultures, LDH release was measured for 48 h following various experimental conditions as described in the methods. Our results showed that IPC reduced cell death to 45 ± 2% compared to 75 ± 2% in the absence of preconditioning (n = 24, p<0.01; 100% is maximal cell death; Fig. 3). To demonstrate whether or not the inhibition of COX-2 activity could abolish ischemic preconditioning in cell cultures, the COX-2 inhibitor NS-398 (50 μM) was applied for 24 h of reperfusion prior to lethal ischemia. The dosage of NS-398 was selected based on effective concentration response (from 1 μM to 50 μM, data not shown). Remarkably, treatment with the COX-2 inhibitor reduced neuronal protection following ischemic preconditioning. The LDH assay revealed that COX-2 inhibition with NS-398 yielded a significant increase in cell death compared to ischemic preconditioning (63 ± 3% vs. 45 ± 2%; n =20, p<0.05; Fig. 3). NS-398 itself after IPC treatment had no effect on neuronal cell death. These results clearly suggest a requirement for COX-2 activation during the late phase of preconditioning.
Fig.3.
COX-2 inhibition reduced IPC-mediated neuroprotection in mixed cortical neuron/astrocyte cell cultures. Histogram representing cell death measured by LDH release at 48 h of reperfusion after OGD (4 h) injury. ** p<0.01 compared with control, # p<0.05 compared with OGD and & p<0.05 compared with IPC.
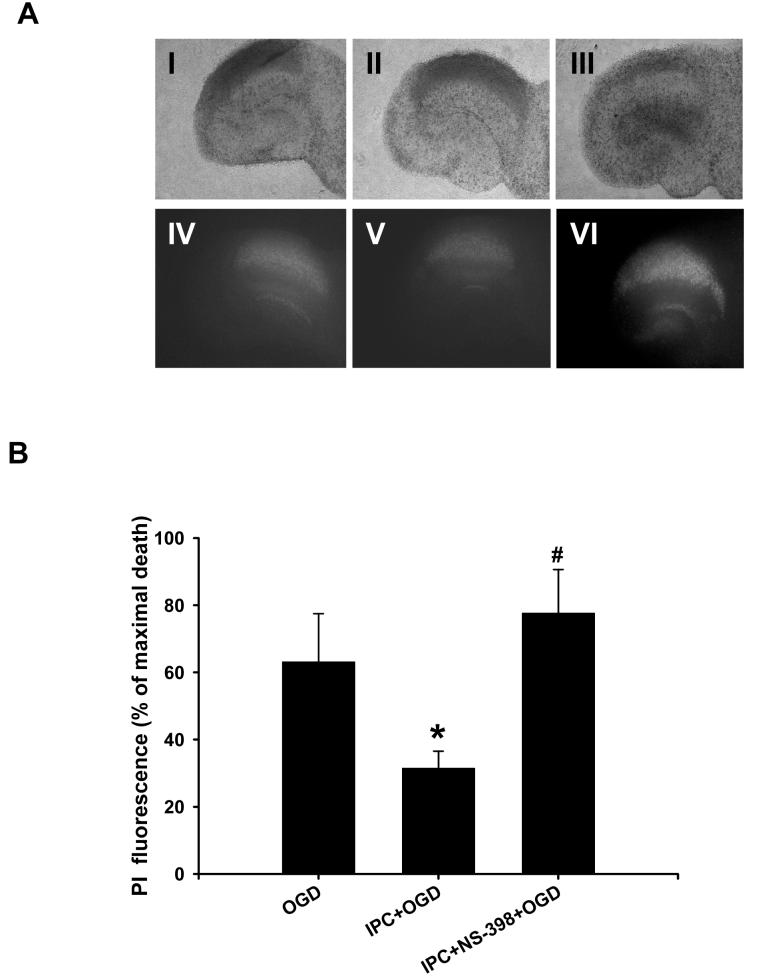
According to our previous report (Raval et al., 2003), ischemic preconditioning also induced neuroprotection in hippocampal organotypic slice cultures. To determine the potential role of COX-2 in ischemic preconditioning, slices were exposed to NS-398 (50 μM) for 48 h of reperfusion after IPC (15 min of oxygen/glucose deprivation). Cell death was measured by staining the slices with propidium iodide, a dye that labels dead cells (Fig. 4A). In the CA1 region of hippocampus, this assay revealed 31 ± 5% of maximal cell death after lethal ischemia following preconditioning and 78 ± 1% following preconditioning and NS-398 treatment (n= 8, p<0.05, Fig. 4B). These results support an important neuroprotective role for COX-2 activity following ischemic preconditioning in both cell cultures and slice cultures.
Fig.4.
COX-2 inhibition abolished IPC-mediated neuroprotection in organotypic hippocampal slices. (A) Bright field (I,II,III) and PI images (IV,V,VI) were taken at 48 h of reperfusion after OGD injury. (I,IV) OGD, (II,V) IPC, and (III,VI) NS-398 treatment for 48 h after IPC (Magnification: 4 X). (B) Histogram representing CA1 neuronal damage measured by PI fluorescence intensity. *p<0.05 compared with OGD, # p<0.05 compared with IPC.
εPKC activation induces COX-2 expression in cell cultures
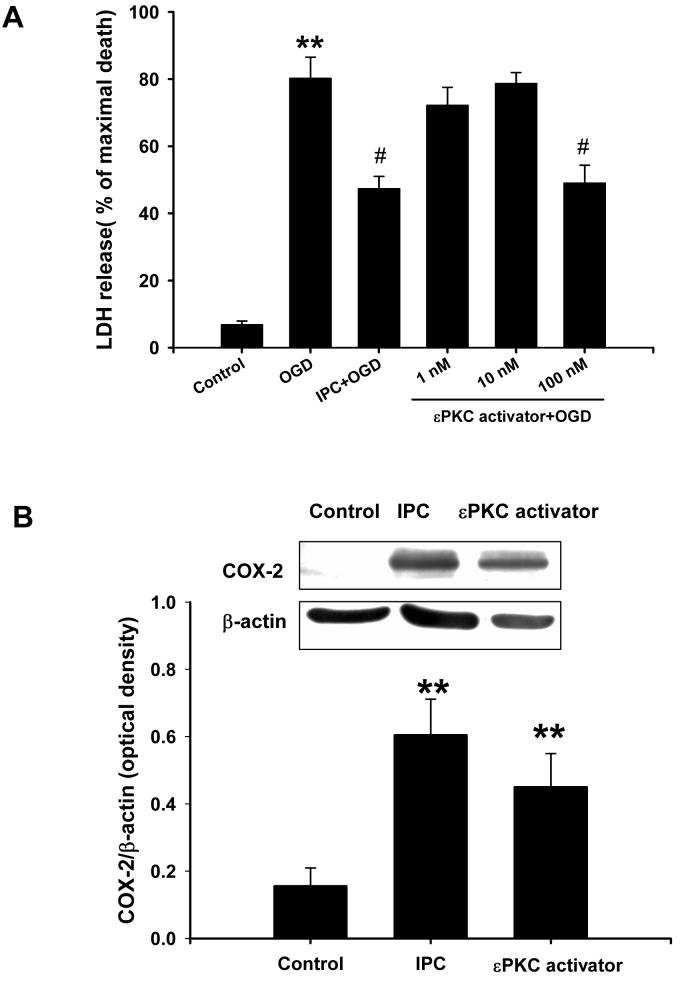
Our previous research demonstrated that εPKC plays a key role in induction of ischemic preconditioning in slice cultures (Raval et al., 2003, Lange-Asschenfeldt et al., 2004). In fact, specific activation of εPKC produced robust neuroprotection against lethal ischemia--a phenomenon we refer to as pharmacological preconditioning (PPC). Based on these findings, here we examined the role of εPKC as an upstream regulator of COX-2 in ischemic and pharmacological preconditioning in cell cultures. Indeed, εPKC activation induces neuroprotection in cell cultures. We demonstrated this by exposing cell cultures to the εPKC-specific activating peptide (1 h, ψεRACK at 1, 10 and 100 nM). Of these, only 100 nM ψεRACK conferred neuroprotection. The results of the LDH assay following lethal ischemia 48 h after εPKC activation or ischemic preconditioning were respectively 47 ± 6% and 42 ± 4% compared to 80 ± 6% of maximal cell death following lethal ischemia alone (n=12 in each group; p<0.05 vs. lethal ischemia; Fig.5A).
Fig.5.
εPKC activation induced IPC-mimicking neuroprotection and COX-2 expression in mixed cortical neuron/astrocyte cell cultures. (A) Histogram representing cell death measured by LDH release at 48 h of reperfusion after OGD (4 h) injury. Note that the IPC (1 h) or εPKC-activating peptide (ψεRACK at 100 nM, 1 h) treatment 48 h prior to OGD (4 h) resulted in cell protection. (B) Representative western blots of COX-2 and β-actin protein at 24 h of reperfusion after 1 h of IPC or 1 h of εPKC- activating peptide treatment. Data are the mean ± S.E.M of normalized densitometry measurements from western blots of COX-2 compared with β-actin (n=5). ** p<0.01 compared with control, # p<0.05 compared with OGD.
Next, we asked if εPKC activation induces COX-2 expression as in ischemic preconditioning. COX-2 expression was examined by western blotting 24 h after 1 h of εPKC activation. These results showed that εPKC activation induced COX-2 expression in a manner similar to ischemic preconditioning (Fig. 5B). These data support the hypothesis that COX-2 is one of the downstream effectors of the εPKC signaling pathway for neuroprotection.
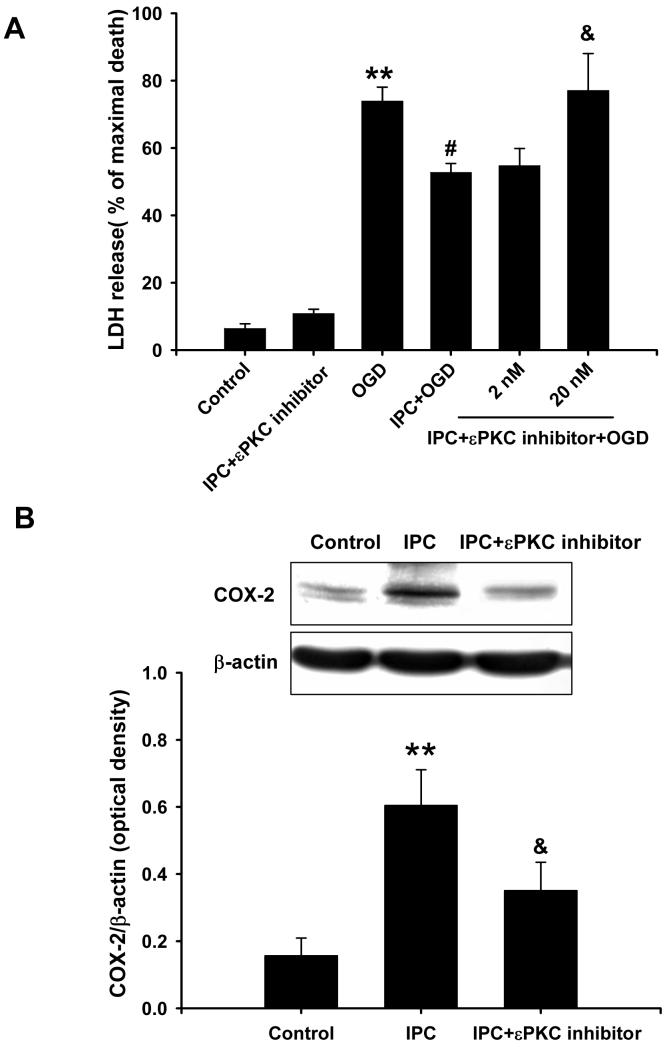
To further characterize this signaling pathway, we inhibited εPKC activation using a εPKC-specific inhibitory peptide (εV1-2 at 2 and 20 nM) during ischemic preconditioning and 48 h of reperfusion in cell cultures. Ischemic preconditioning in the presence of the εPKC inhibitory peptide (20 nM) lacked the neuroprotection conferred by ischemic preconditioning alone (77 ± 11 % vs. 53 ± 3% of maximal cell death; n=12; p<0.05; Fig. 6A). In fact, cell death was indistinguishable from the lethal ischemia control group (74 ± 4%). Additionally, the εPKC-specific inhibitory peptide reduced COX-2 induction by IPC (Fig. 6B). Overall, these results clearly demonstrated that εPKC activation is a key upstream regulator of COX-2 induction resulting in neuroprotection.
Fig.6.
Inhibition of εPKC activation reduced IPC-mediated neuroprotection and COX-2 expression in mixed cortical neuron/astrocyte cell cultures. (A) Histogram representing cell death, measured by LDH release at 48 h of reperfusion after OGD (4 h) injury. Note the loss of cell protection due to inhibition of εPKC activation using εPKC inhibitory peptide (εV1-2 at 20 nM) during IPC and 48 h of reperfusion. (B) Representative western blots of COX-2 and β-actin protein at 24 h of reperfusion after IPC. Data are the mean ± S.E.M. of normalized densitometry measurements from western blots of COX-2 compared with β-actin (n=5). ** p<0.01 compared with control, # p<0.05 compared with OGD, and & p<0.05 compared with IPC.
εPKC activation induces COX-2 expression via ERK1/2 phosphorylation
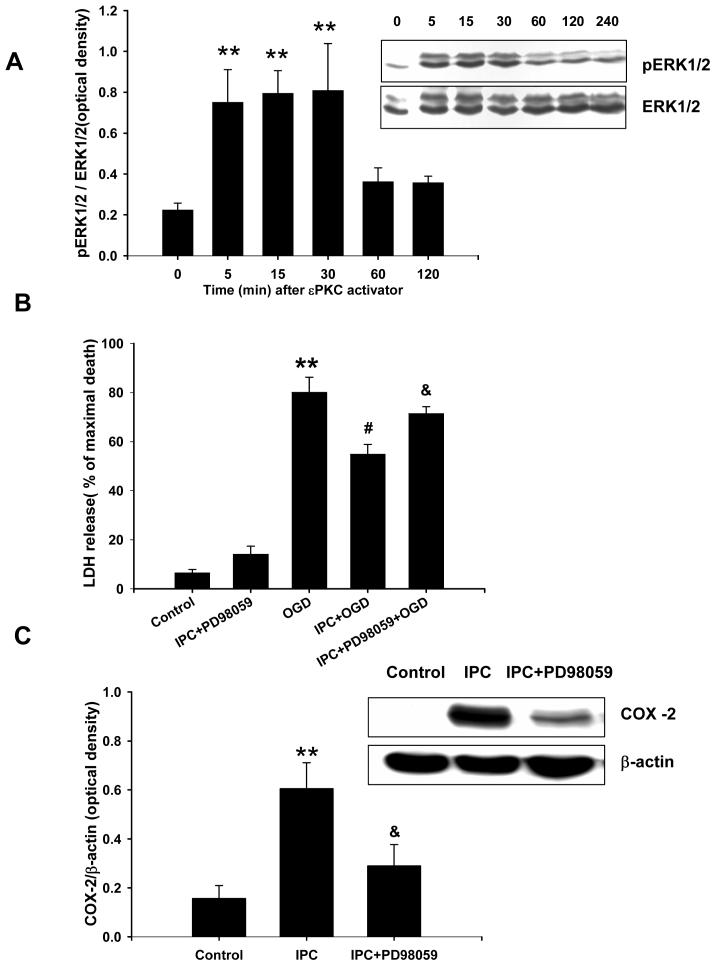
In slice cultures, previous studies have shown that ischemic preconditioning activates εPKC which in turn leads to phosphorylation of ERK1/2 (Lange-Asschenfeldt et al., 2004). We asked whether direct εPKC activation leads to phosphorylation of ERK1/2. We exposed cell cultures to the εPKC-specific activating peptide (ψεRACK, 100 nM), and cells were collected at various intervals (5, 15, 30, 60 and 120 min) for an assay that detects phosphorylated ERK1/2. These results showed a prominent increase in phosphorylated ERK1/2 within 5 min of εPKC activator application and persisted for 30 min (Fig. 7A).
Fig.7.
εPKC activation induced ERK1/2 phosphorylation and Inhibition of ERK1/2 phosphorylation reduced IPC-mediated neuroprotection and COX-2 expression in mixed cortical neuron/astrocyte cell cultures. (A) Representative western blots of ERK1/2 protein at 5, 15, 60, 120 and 240 min after 1 h of εPKC-activating peptide treatment (ψεRACK at 100 nM). Data are the mean ± S.E.M of normalized densitometry measurements from western blots of pERK1/2 compared with ERK1/2 (n=5). (B) Histogram representing cell death, measured by LDH release at 48 h of reperfusion after OGD (4 h) injury. Note the loss of cell protection due to inhibition of ERK1/2 using PD98059 (at 10 μM) during IPC and 48 h of reperfusion. Representative western blots of COX-2 and β-actin protein at 24 h of reperfusion after 1 h of IPC. (C) Data are the mean ± SEM of normalized densitometry measurements from western blots of COX-2 compared with β-actin (n=5). ** p<0.01 compared with control, # p<0.05 compared with OGD, and & p<0.05 compared with IPC.
Our next goal was to delineate the signaling pathway triggered by ischemic preconditioning and leading to expression of the mediators of neuroprotection. We have already shown that ischemic preconditioning activates εPKC, phosphorylates ERK1/2, and induces COX-2 expression. We asked whether blockade of ERK1/2 phosphorylation using an inhibitor (PD98059, 10 μM) of mitogen-activated protein kinase-kinase (MAPK-K), prevented ischemic preconditioning in cell cultures. The LDH cell death assay revealed that PD98059 prevented neuroprotection afforded by ischemic preconditioning (71 ± 3% vs. 52 ± 4% of maximal cell death (n=20, p<0.05; Fig. 7B). Parallel to this finding, we found that inhibition of ERK1/2 phosphorylation with PD98059 after ischemic preconditioning also reduced COX-2 expression (Fig. 7C). These results suggested that εPKC → MAPK-K → ERK1/2 pathway is involved in ischemic preconditioning via COX-2 expression.
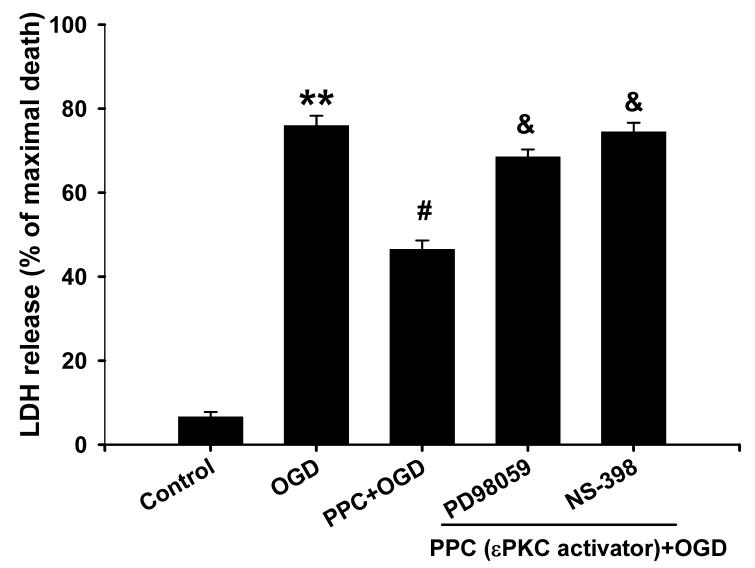
Finally, we tested the hypothesis that pharmacological preconditioning via direct εPKC activation requires ERK1/2 phosphorylation and COX-2 induction. The MAPK-K inhibitor PD98059 was applied during 1 h of pharmacological preconditioning with the εPKC activator ψεRACK and 48 h later ischemia was induced with oxygen/glucose deprivation. PD98059 significantly decreased neuroprotection conferred by pharmacological preconditioning (68 ± 2% vs. 46 ± 2% of maximal cell death; PD98059+PPC vs. PPC; n=16 p<0.05; Fig. 8). Inhibition of COX-2 with NS-398 for 24 h after pharmacological preconditioning also significantly diminished neuroprotection (74 ± 2% of maximal cell death; n=16, p<0.05; Fig. 8). Based on these results, we propose that ischemic preconditioning via εPKC activation requires phosphorylation of ERK1/2 and induction COX-2.
Fig.8.
εPKC-mediated neuroprotection prevented by inhibition of ERK1/2 and COX-2 activation. 10 μM PD98059 was treated during 1 h of εPKC-activating peptide treatment (ψεRACK at 100 nM) and 48 h of reperfusion, or NS-398 was treated for 24 h of reperfusion prior to ischemia after 1 h of εPKC-activating peptide treatment. Then, OGD was done, and 48 h later, LDH assay was measured for cell death. PPC; pharmacological preconditioning. ** p<0.01 compared with control, # p<0.05 compared with OGD, and & p<0.05 compared with PPC.
DISCUSSION
In the present study we demonstrated that (1) ischemic preconditioning induced COX-2 expression in cortical neuron/astrocyte cell cultures and (2) inhibition of COX-2 activity resulted in reduction of ischemic preconditioning in both cell culture and organotypic hippocampal slice culture. In harmony with our previous findings in slice cultures (Raval et al., 2003, Lange-Asschenfeldt et al., 2004), the current study also emphasized a key role for εPKC activation in ischemic preconditioning in cell cultures. Activation of εPKC resulted in ERK 1/2 phosphorylation, COX-2 expression and neuroprotection in cell cultures. Inhibition of εPKC and ERK1/2 abolished induction of COX-2 expression and neuroprotection by ischemic preconditioning. Taken together, these results support the hypothesis that COX-2 induction via the εPKC → MAPK-K → ERK1/2 signaling pathway is necessary for the late phase of ischemic preconditioning.
Cyclooxygenase-2, the rate-limiting enzyme for prostanoid synthesis, has been implicated in the pathogenesis of a wide variety of neurological diseases, including ischemic stroke (Minghetti, 2004, Iadecola and Gorelick, 2005). In contrast, the present study suggests a neuroprotective role for COX-2 against ischemic injury. Our results demonstrated that ischemic preconditioning induced COX-2 expression at 15 h and sustained for 36 h of reperfusion. Surprisingly, while various other effectors of preconditioning were demonstrated to be activated in shortly after IPC, our study demonstrated delayed activation of COX-2. Consistent with our observations, COX-2 expression has been demonstrated to occur 24 h after IPC induction in the heart (Shinmura et al., 2000). The delayed COX-2 expression after IPC emphasizes the need for de novo protein synthesis. Based on our data demonstrating delayed COX-2 activation, we conjecture that COX-2 activity or COX-2-derived prostaglandins might be required for induction of late protective mechanisms of ischemic preconditioning.
Despite growing evidence for an essential role of COX-2 in preconditioning, the nature of the subsequent signaling events is less well understood. Numerous studies have shown COX-2 activation by PKC in various cell types (Kim and Chun, 2003, Rodriguez et al., 2006). The PKC family is comprised of 12 isozymes and different PKC isozymes play diverse cellular functions. In this respect, it is essential to identify the specific isozyme involved in COX-2 activation following ischemic preconditioning in neurons. Recent evidence in heart demonstrated that εPKC activation resulted in upregulation of COX-2 and cardioprotection (Dawn and Bolli, 2002, Xuan et al., 2005). εPKC has been demonstrated to play a key role in the induction of preconditioning via NMDA or adenosine receptor activation (Di-Capua et al., 2003, Raval et al., 2003, Lange-Asschenfeldt et al., 2004). Consistent with this, the present study demonstrated εPKC activation after ischemic preconditioning in mixed cortical neuron/astrocyte cell cultures. Further, activation of εPKC with isozyme-specific activator led to neuroprotection against subsequent ischemic injury. Interestingly, preconditioning via direct εPKC activation resulted in increased COX-2 expression, suggesting a link between εPKC and COX-2.
The signaling pathways responsible for downstream regulation of εPKC include: the Src family of protein tyrosine kinase (Bolli et al., 1998, Bolli, 2000), p38 (Weber et al., 2005), MEK1/2- ERK1/2 (Xuan et al., 2005), Akt , NF-κB and AP-1 (Li et al., 2000) in heart and neurons. Of the various regulators of COX-2, the p38, ERK1/2 (Blanco et al., 1995) NF-κB and AP-1 pathways (von Knethen et al., 1999, Allport et al., 2000) are well-known upstream mediators of COX-2 induction in inflammation and carcinogenesis (Fan et al., 2001, Jung et al., 2003, Chen et al., 2005). Another related study demonstrated that inflammatory agent-induced COX-2 expression was regulated by εPKC → ERK1/2 activation in cortical neurons (Wu et al., 2006). In harmony with this study, our study using the MAPK-K inhibitor PD98059 indicated a role for εPKC → ERK1/2 in COX-2 expression. These results were confirmed by the observation of increased phosphorylation of ERK1/2 following activation of εPKC. We also observed that the MAPK-K inhibitor PD98059 reduction of COX-2 induction by ischemic preconditioning. In support of these findings, cortical spreading depression-induced preconditioning increased ERK1/2 phosphorylation and COX-2 expression (Horiguchi et al., 2005). Although other components of the signaling pathways for activation of εPKC → ERK1/2 → COX-2 remain undefined, an essential feature of the activation of COX-2 is its regulation by NF-κB. NF-κB is one of the transcription factors has been shown to play an essential role in cardioprotection (Xuan et al., 1999, Zhao and Kukreja, 2002) and brain tolerance (Blondeau et al., 2001, Digicaylioglu and Lipton, 2001). It is well known that the NF-κB DNA binding site is located in the COX-2 promoter construct. Strong NF-κB DNA binding is fundamental in COX-2 expression in neurodegenerative disorders (Lukiw and Bazan, 1998). Shinmura et al. have reported that ischemic preconditioning upregulated COX-2 expression via PKC → Src PTK → NF-κB in heart (Shinmura et al., 2002b). Thus, based on these studies, it is likely that COX-2 would be induced by NF-κB activation through εPKC → ERK1/2 signaling pathway.
Although the exact mechanisms by which ischemic preconditioning mediates neuroprotection via COX-2 induction remain unknown, it is believed that some of prostaglandins derived from COX-2 activity are involved. McCullough et al. suggested that PGE2 mediates neuroprotection via PGE2/ prostaglandin E2 receptor (EP2) /cAMP-dependent manner in cerebral ischemia (McCullough et al., 2004). Also, in our previous in vitro studies, PGE2 protected inflammation-mediated neuronal cell death via a cAMP-dependent manner (Kim et al., 2002b). 15d –prostaglandin J2, a prostaglandin D2 derivative, protects neurons against ischemia-reperfusion injury through a peroxisome proliferator activated receptor-γ (PPAR-γ)-dependent manner (Lin et al., 2006). Furthermore, Horiguchi et al have reported that at 15d –prostaglandin J2 production was increased as well as COX-2 expression during cortical spreading depression-induced tolerance (Horiguchi et al., 2006). However, recently, COX-2 derived PGE2 induced neurotoxicity by Ca2+ dysregulation via EP2 receptor in ischemic brain injury (Kawano et al., 2006). Although the effect of COX-2-derived PGE2 on neuroprotection or neurotoxicity is still controversial, it is likely to be dependent on different PG products and on different PG receptor types. Thus, the downstream effectors of COX-2 expression after ischemic preconditioning need to be further studied.
CONCLUSION
In conclusion, ischemic preconditioning induced COX-2 expression in cortical neurons and blockade of COX-2 activity abolished neuroprotection in mixed cortical neuron/astrocyte cell cultures and in organotypic hippocampal slice cultures. εPKC activation induced phosphorylation of ERK1/2 and COX-2 expression resulting in neuroprotection against ischemia. Thus, COX-2 induction via εPKC and ERK1/2 activation is required for neuroprotection via ischemic preconditioning.
Acknowledgments
This study was supported by PHS grants NS34773, NS054147, NS045676, NS05820, and AHA Florida& Puerto Rico Affiliate grant 0525331B. We thank Dr. Brant Watson for critical reading of this manuscript.
Abbreviations
- BSA
bovine serum albumin
- Cell cultures
mixed cortical neuron/astrocyte cell cultures
- COX-2
cyclooxygenase-2
- ERK1/2
extracellular signal regulated kinase 1/2
- FBS
fetal bovine serum
- GFAP
glial fibrillary acidic protein
- HBSS
hank's balanced salt solution
- IPC
ischemic preconditioning
- LDH
lactate dehydrogenase
- LSM
laser scanning microscopy
- MAPK-K
mitogen-activated protein kinase- kinase
- MEM
minimum essential medium
- NeuN
neuron nuclear
- NF-κB
nuclear factor –kappa B
- NOS
nitric oxide synthase
- NS-398
N-[2-(cyclohexyloxy)-4-nitrophenyl]-methanesulfonamide
- OGD
oxygen/glucose deprivation
- PBS-T
phosphate-buffered saline containing 0.4% triton X-100
- PD98059
2'-amino-3'-methoxyflavone
- PG
prostaglandin
- PI
propidium iodide
- PKC
protein kinase C
- PPC
pharmacological preconditioning
- PTK
protein tyrosine kinase
- ROI
relative optical intensity
- Slice cultures
organotypic hippocampal slice cultures
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J Biol Chem. 1999;274:5038–5046. doi: 10.1074/jbc.274.8.5038. [DOI] [PubMed] [Google Scholar]
- Allport VC, Slater DM, Newton R, Bennett PR. NF-kappaB and AP-1 are required for cyclo-oxygenase 2 gene expression in amnion epithelial cell line (WISH) Mol Hum Reprod. 2000;6:561–565. doi: 10.1093/molehr/6.6.561. [DOI] [PubMed] [Google Scholar]
- Baik EJ, Kim EJ, Lee SH, Moon C. Cyclooxygenase-2 selective inhibitors aggravate kainic acid induced seizure and neuronal cell death in the hippocampus. Brain Res. 1999;843:118–129. doi: 10.1016/s0006-8993(99)01797-7. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Fletcher BS, Herschman HR, Mukherjee PK. Platelet-activating factor and retinoic acid synergistically activate the inducible prostaglandin synthase gene. Proc Natl Acad Sci U S A. 1994;91:5252–5256. doi: 10.1073/pnas.91.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco A, Habib A, Levy-Toledano S, Maclouf J. Involvement of tyrosine kinases in the induction of cyclo-oxygenase-2 in human endothelial cells. Biochem J. 1995;312(Pt 2):419–423. doi: 10.1042/bj3120419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondeau N, Widmann C, Lazdunski M, Heurteaux C. Activation of the nuclear factor-kappaB is a key event in brain tolerance. J Neurosci. 2001;21:4668–4677. doi: 10.1523/JNEUROSCI.21-13-04668.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- Bolli R, Dawn B, Tang XL, Qiu Y, Ping P, Xuan YT, Jones WK, Takano H, Guo Y, Zhang J. The nitric oxide hypothesis of late preconditioning. Basic Res Cardiol. 1998;93:325–338. doi: 10.1007/s003950050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LC, Chen BK, Chang WC. Activating protein 1-mediated cyclooxygenase-2 expression is independent of N-terminal phosphorylation of c-Jun. Mol Pharmacol. 2005;67:2057–2069. doi: 10.1124/mol.104.010900. [DOI] [PubMed] [Google Scholar]
- Dawn B, Bolli R. Role of nitric oxide in myocardial preconditioning. Ann N Y Acad Sci. 2002;962:18–41. doi: 10.1111/j.1749-6632.2002.tb04053.x. [DOI] [PubMed] [Google Scholar]
- Di-Capua N, Sperling O, Zoref-Shani E. Protein kinase C-epsilon is involved in the adenosine-activated signal transduction pathway conferring protection against ischemia-reperfusion injury in primary rat neuronal cultures. J Neurochem. 2003;84:409–412. doi: 10.1046/j.1471-4159.2003.01563.x. [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- Fan XM, Wong BC, Lin MC, Cho CH, Wang WP, Kung HF, Lam SK. Interleukin-1beta induces cyclo-oxygenase-2 expression in gastric cancer cells by the p38 and p44/42 mitogen-activated protein kinase signaling pathways. J Gastroenterol Hepatol. 2001;16:1098–1104. doi: 10.1046/j.1440-1746.2001.02593.x. [DOI] [PubMed] [Google Scholar]
- Gendron TF, Brunette E, Tauskela JS, Morley P. The dual role of prostaglandin E(2) in excitotoxicity and preconditioning-induced neuroprotection. Eur J Pharmacol. 2005;517:17–27. doi: 10.1016/j.ejphar.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Zulueta M, Feldman AB, Klesse LJ, Kalb RG, Dillman JF, Parada LF, Dawson TM, Dawson VL. Requirement for nitric oxide activation of p21(ras)/extracellular regulated kinase in neuronal ischemic preconditioning. Proc Natl Acad Sci U S A. 2000;97:436–441. doi: 10.1073/pnas.97.1.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi T, Snipes JA, Kis B, Shimizu K, Busija DW. The role of nitric oxide in the development of cortical spreading depression-induced tolerance to transient focal cerebral ischemia in rats. Brain Res. 2005;1039:84–89. doi: 10.1016/j.brainres.2005.01.047. [DOI] [PubMed] [Google Scholar]
- Horiguchi T, Snipes JA, Kis B, Shimizu K, Busija DW. Cyclooxygenase-2 mediates the development of cortical spreading depression-induced tolerance to transient focal cerebral ischemia in rats. Neuroscience. 2006 doi: 10.1016/j.neuroscience.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Hoshida S, Kuzuya T, Fuji H, Yamashita N, Oe H, Hori M, Suzuki K, Taniguchi N, Tada M. Sublethal ischemia alters myocardial antioxidant activity in canine heart. Am J Physiol. 1993;264:H33–39. doi: 10.1152/ajpheart.1993.264.1.H33. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Gorelick PB. The Janus face of cyclooxygenase-2 in ischemic stroke: shifting toward downstream targets. Stroke. 2005;36:182–185. doi: 10.1161/01.STR.0000153797.33611.d8. [DOI] [PubMed] [Google Scholar]
- Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. Faseb J. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Kwon KJ, Park JY, Lee SH, Moon CH, Baik EJ. Effects of peroxisome proliferator-activated receptor agonists on LPS-induced neuronal death in mixed cortical neurons: associated with iNOS and COX-2. Brain Res. 2002a;941:1–10. doi: 10.1016/s0006-8993(02)02480-0. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Kwon KJ, Park JY, Lee SH, Moon CH, Baik EJ. Neuroprotective effects of prostaglandin E2 or cAMP against microglial and neuronal free radical mediated toxicity associated with inflammation. J Neurosci Res. 2002b;70:97–107. doi: 10.1002/jnr.10373. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Lee JE, Kwon KJ, Lee SH, Moon CH, Baik EJ. Differential roles of cyclooxygenase isoforms after kainic acid-induced prostaglandin E(2) production and neurodegeneration in cortical and hippocampal cell cultures. Brain Res. 2001;908:1–9. doi: 10.1016/s0006-8993(01)02432-5. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Chun JS. Protein kinase C alpha and zeta regulate nitric oxide-induced NF-kappa B activation that mediates cyclooxygenase-2 expression and apoptosis but not dedifferentiation in articular chondrocytes. Biochem Biophys Res Commun. 2003;303:206–211. doi: 10.1016/s0006-291x(03)00305-x. [DOI] [PubMed] [Google Scholar]
- Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987;20:83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- Lange-Asschenfeldt C, Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Perez-Pinzon MA. Epsilon protein kinase C mediated ischemic tolerance requires activation of the extracellular regulated kinase pathway in the organotypic hippocampal slice. J Cereb Blood Flow Metab. 2004;24:636–645. doi: 10.1097/01.WCB.0000121235.42748.BF. [DOI] [PubMed] [Google Scholar]
- LaPointe MC, Mendez M, Leung A, Tao Z, Yang XP. Inhibition of cyclooxygenase-2 improves cardiac function after myocardial infarction in the mouse. Am J Physiol Heart Circ Physiol. 2004;286:H1416–1424. doi: 10.1152/ajpheart.00136.2003. [DOI] [PubMed] [Google Scholar]
- Li RC, Ping P, Zhang J, Wead WB, Cao X, Gao J, Zheng Y, Huang S, Han J, Bolli R. PKCepsilon modulates NF-kappaB and AP-1 via mitogen-activated protein kinases in adult rabbit cardiomyocytes. Am J Physiol Heart Circ Physiol. 2000;279:H1679–1689. doi: 10.1152/ajpheart.2000.279.4.H1679. [DOI] [PubMed] [Google Scholar]
- Lin TN, Cheung WM, Wu JS, Chen JJ, Lin H, Liou JY, Shyue SK, Wu KK. 15d-prostaglandin J2 protects brain from ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:481–487. doi: 10.1161/01.ATV.0000201933.53964.5b. [DOI] [PubMed] [Google Scholar]
- Ludwig LM, Weihrauch D, Kersten JR, Pagel PS, Warltier DC. Protein kinase C translocation and Src protein tyrosine kinase activation mediate isoflurane-induced preconditioning in vivo: potential downstream targets of mitochondrial adenosine triphosphate-sensitive potassium channels and reactive oxygen species. Anesthesiology. 2004;100:532–539. doi: 10.1097/00000542-200403000-00011. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Bazan NG. Strong nuclear factor-kappaB-DNA binding parallels cyclooxygenase-2 gene transcription in aging and in sporadic Alzheimer's disease superior temporal lobe neocortex. J Neurosci Res. 1998;53:583, 592. doi: 10.1002/(SICI)1097-4547(19980901)53:5<583::AID-JNR8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci U S A. 1999;96:272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, Breyer RM, Andreasson K. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci. 2004;24:257–268. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63:901–910. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Uchimura K, Zhu RL, Nagayama T, Rose ME, Stetler RA, Isakson PC, Chen J, Graham SH. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci U S A. 1998;95:10954–10959. doi: 10.1073/pnas.95.18.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Perez-Pinzon MA. Epsilon PKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J Neurosci. 2003;23:384–391. doi: 10.1523/JNEUROSCI.23-02-00384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JA, De la Cerda P, Collyer E, Decap V, Vio CP, Velarde V. Cyclooxygenase-2 induction by bradykinin in aortic vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;290:H30–36. doi: 10.1152/ajpheart.00349.2005. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kitagawa K, Yamagata K, Takemiya T, Tanaka S, Omura-Matsuoka E, Sugiura S, Matsumoto M, Hori M. Amelioration of hippocampal neuronal damage after transient forebrain ischemia in cyclooxygenase-2-deficient mice. J Cereb Blood Flow Metab. 2004;24:107–113. doi: 10.1097/01.WCB.0000100065.36077.4A. [DOI] [PubMed] [Google Scholar]
- Shinmura K, Bolli R, Liu SQ, Tang XL, Kodani E, Xuan YT, Srivastava S, Bhatnagar A. Aldose reductase is an obligatory mediator of the late phase of ischemic preconditioning. Circ Res. 2002a;91:240–246. doi: 10.1161/01.res.0000029970.97247.57. [DOI] [PubMed] [Google Scholar]
- Shinmura K, Tang XL, Wang Y, Xuan YT, Liu SQ, Takano H, Bhatnagar A, Bolli R. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci U S A. 2000;97:10197–10202. doi: 10.1073/pnas.97.18.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Xuan YT, Tang XL, Kodani E, Han H, Zhu Y, Bolli R. Inducible nitric oxide synthase modulates cyclooxygenase-2 activity in the heart of conscious rabbits during the late phase of ischemic preconditioning. Circ Res. 2002b;90:602–608. doi: 10.1161/01.res.0000012202.52809.40. [DOI] [PubMed] [Google Scholar]
- Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- Takeda K, Lin J, Okubo S, Akazawa-Kudoh S, Kajinami K, Kanemitsu S, Tsugawa H, Kanda T, Matsui S, Takekoshi N. Transient glucose deprivation causes upregulation of heme oxygenase-1 and cyclooxygenase-2 expression in cardiac fibroblasts. J Mol Cell Cardiol. 2004;36:821–830. doi: 10.1016/j.yjmcc.2004.03.008. [DOI] [PubMed] [Google Scholar]
- von Knethen A, Callsen D, Brune B. Superoxide attenuates macrophage apoptosis by NF-kappa B and AP-1 activation that promotes cyclooxygenase-2 expression. J Immunol. 1999;163:2858–2866. [PubMed] [Google Scholar]
- Weber NC, Toma O, Wolter JI, Obal D, Mullenheim J, Preckel B, Schlack W. The noble gas xenon induces pharmacological preconditioning in the rat heart in vivo via induction of PKC-epsilon and p38 MAPK. Br J Pharmacol. 2005;144:123–132. doi: 10.1038/sj.bjp.0706063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HH, Hsieh WS, Yang YY, Tsai MC. Lipoteichoic acid induces prostaglandin E(2) release and cyclooxygenase-2 synthesis in rat cortical neuronal cells: involvement of PKCepsilon and ERK activation. Life Sci. 2006;79:272–280. doi: 10.1016/j.lfs.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Xu GP, Dave KR, Vivero R, Schmidt-Kastner R, Sick TJ, Perez-Pinzon MA. Improvement in neuronal survival after ischemic preconditioning in hippocampal slice cultures. Brain Res. 2002;952:153–158. doi: 10.1016/s0006-8993(02)02988-8. [DOI] [PubMed] [Google Scholar]
- Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Messing RO, Bolli R. Role of the protein kinase C-epsilon-Raf-1-MEK-1/2-p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase-2 after ischemic preconditioning. Circulation. 2005;112:1971–1978. doi: 10.1161/CIRCULATIONAHA.105.561522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan YT, Tang XL, Banerjee S, Takano H, Li RC, Han H, Qiu Y, Li JJ, Bolli R. Nuclear factor-kappaB plays an essential role in the late phase of ischemic preconditioning in conscious rabbits. Circ Res. 1999;84:1095–1109. doi: 10.1161/01.res.84.9.1095. [DOI] [PubMed] [Google Scholar]
- Yasojima K, Schwab C, McGeer EG, McGeer PL. Distribution of cyclooxygenase-1 and cyclooxygenase-2 mRNAs and proteins in human brain and peripheral organs. Brain Res. 1999;830:226–236. doi: 10.1016/s0006-8993(99)01389-x. [DOI] [PubMed] [Google Scholar]
- Zhao TC, Kukreja RC. Late preconditioning elicited by activation of adenosine A(3) receptor in heart: role of NF- kappa B, iNOS and mitochondrial K(ATP) channel. J Mol Cell Cardiol. 2002;34:263–277. doi: 10.1006/jmcc.2001.1510. [DOI] [PubMed] [Google Scholar]