Abstract
Smaller-sized fungal fragments (<1 μm) may contribute to mold-related health effects. Previous laboratory-based studies have shown that the number concentration of fungal fragments can be up to 500 times higher than that of fungal spores, but this has not yet been confirmed in a field study due to lack of suitable methodology. We have recently developed a field-compatible method for the sampling and analysis of airborne fungal fragments. The new methodology was utilized for characterizing fungal fragment exposures in mold-contaminated homes selected in New Orleans, Louisiana and Southern Ohio. Airborne fungal particles were separated into three distinct size fractions: (i) >2.25 μm (spores); (ii) 1.05–2.25 μm (mixture); and (iii) < 1.0 μm (submicrometer-sized fragments). Samples were collected in five homes in summer and winter and analyzed for (1→3)-β-D-glucan. The total (1→3)-β-D-glucan varied from 0.2 to 16.0 ng m−3. The ratio of (1→3)-β-D-glucan mass in fragment size fraction to that in spore size fraction (F/S) varied from 0.011 to 2.163. The mass ratio was higher in winter (average = 1.017) than in summer (0.227) coinciding with a lower relative humidity in the winter. Assuming a mass-based F/S-ratio=1 and the spore size = 3 μm, the corresponding number-based F/S-ratio (fragment number/spore number) would be 103 and 106, for the fragment sizes of 0.3 and 0.03 μm, respectively. These results indicate that the actual (field) contribution of fungal fragments to the overall exposure may be very high, even much greater than that estimated in our earlier laboratory-based studies.
Keywords: mold, beta-glucan, particle size, exposure assessment
1. Introduction
Moisture damage is common around the world and has been reported for various kinds of buildings such as homes, schools, offices, and even hospitals. If the moisture damage is not observed or remediated, it may be conductive to microbial growth. Several epidemiological studies have found an association between dampness or visible mold and respiratory illness of children and adults as summarized by the Institute of Medicine (2004). It is notable that majority of the previous epidemiological studies have relied on either self-reported mold and dampness or surveyor-assessed moisture and visible mold. However, attempts to correlate health outcomes with spore counts have not indicated strong associations. Furthermore, several field studies have shown that the concentrations of airborne fungal spores in mold problem buildings are not necessarily higher than in non-problem ones (Strachan et al., 1990; Nevalainen et al., 1991; Garrett et al., 1998; Chew et al., 2003). This indicates that spore concentrations may not be an adequate measure for fungal exposures.
The existence of larger hyphal fragments in the ambient air has been recognized for some time (Glikson et al., 1995; Li and Kendrick, 1996), but has so far been overlooked when assessing exposures in moldy buildings. Hyphal fragments have been shown to represent 6–56% of the total fungal particle counts in field samples based on microscopic sample analysis (Li and Kendrick, 1996; Foto et al., 2005; Green et al., 2005). This method is limited typically to particles >1 μm (Green et al., 2006). Recent laboratory-based studies (Górny et al., 2002 and 2003; Cho et al., 2005) have reported that large quantities of submicrometer-sized fungal and actinomycete fragments (ranging from 30 nm to 1 μm) are released together with intact spores from contaminated surfaces. These studies demonstrated that the number of released fragments was always higher, up to 500 times, than the number of intact spores. Furthermore, the number of spores and fragments did not correlate.
Assessing exposure to smaller-sized fungal fragments may be important for several reasons. Smaller-sized fragments have longer lifetimes in the air compared to larger spores and can penetrate deeply into the alveolar region when inhaled. Fungal fragments have been shown to contain fungal antigens (Górny et al., 2002), mycotoxins (Brasel et al., 2005a, b), and (1→3)-β-D-glucan (Seo et al., 2007). The small size, large quantities, and biological properties of fungal fragments suggest that these particles may potentially contribute to the adverse health effects and raises the need for further characterizations of fungal fragments in moldy buildings. The quantification of fungal fragments, including those of nano-scale sizes, has previously been hindered by the lack of suitable field-compatible sampling and analysis methods.
To our knowledge, there is only one previous study assessing submicrometer-sized fungal fragments in mold-contaminated buildings (Brasel et al., 2005b). Fungal fragments were separated from spores using two filters of decreasing pore sizes placed in a series. While this method allows an efficient separation of submicrometer-sized fragments from intact spores, it is likely to underestimate the amount of submicrometer-sized fragments, as a large proportion of these particles are collected already onto the first filter due to diffusion. Cascade impactors would be an alternative that potentially offers a sharp separation of fragments from spores. Our previous study, however, showed that collection of purified fragments by impactors is challenging because of the spore bounce from upper stages to lower stages (Cho et al., 2005).
We have recently developed a field-compatible method for separation and analysis of airborne submicrometer-sized fungal fragments (Seo et al., 2007). The Fragment Sampling System utilized Sharp-Cut cyclones, which allow minimizing the spore bounce separating the airborne particles into three distinct size fractions, which are subsequently, analyzed for (1→3)-β-D-glucan. This new methodology was utilized for characterizing submicrometer-sized fungal fragments in aerosol samples collected in field conditions in moldy homes.
2. Materials and Methods
2.1 Selection of Homes
The field investigation was conducted in five mold-contaminated single-family houses (Table 1). Three houses, located in New Orleans, Louisiana, were flooded during hurricane Katrina, and were severely mold-contaminated. Two other houses located in Southern Ohio; both had suffered water-damage in the basement and had either water-damage (house 4) or visible mold (house 5). Sampling was performed in all five homes during the summer of 2006 (June–September) and repeated in two homes in the winter (December, 2006–January, 2007).
Table 1.
Summary of the characteristics of the houses.
| House 1 | House 2 | House 3 | House 4 | House 5 | |
|---|---|---|---|---|---|
| Location | New Orleans | New Orleans | New Orleans | Southern Ohio | Southern Ohio |
| Total number of rooms | 7 | 9 | 8 | 3 | 3 |
| Room for sample collection | Living room | Living room | Living room | Basement | Basement |
| Floor material | Wood | Carpet | Wood | Concrete | Concrete |
| Room size (m2) | 26.1 | 29.7 | 46.2 | 133.1 | 35.6 |
| Cause of water damage | Flooding (1.83 m) | Flooding (3.05 m) | Flooding (2.74 m) | Water leakage through wall | Water leakage through wall and window sills |
| Damaged materials | Visible mold on all surfaces | Visible mold on all surfaces | Visible mold on all surfaces | Several spots of water- damage on dry wall (<0.2 m2) | Visible mold on dry wall (~3 m2) |
| Occupied | No | No | No | No | Yes |
2.2 Safety Measures
Prior to field sampling in New Orleans, respirator fit-test was performed for all personnel participating in field sampling. Half mask respirators (North Safety Products, Cranston, RI), safety goggles, gloves, and tyvek suits with hoods (DuPont Tyvek, Wilmington, DE) were worn during sampling.
2.3 Air Sampling
Size-selective sampling of fungal particles was performed using our recently-developed Fragment Sampling System (Seo et al., 2007) that consists of two Sharp-Cut cyclones (BGI Inc., Waltham, MA) and a 25-mm after-filter (gamma-radiated preloaded polycarbonate filters with a pore size of 0.4 μm, SKC Inc., Eighty Four, PA). Airborne particles were separated into three distinct size fractions according to their aerodynamic size: (i) >2.25 μm (spores); (ii) 1.05–2.25 μm (mixture of spores and fragments); and (iii) < 1.0 μm (submicrometer-sized fragments). The collection efficiency of the after-filter was found to be over 99.2% when tested with NaCl aerosol particles in the size range of 0.01–0.3 μm (Seo et al., 2007). The flow-rate of 16.71 min−1 was achieved using a high-volume air sampling pump (Model SP-280, Air Diagnostics and Engineering Inc., Harrison, ME). It was powered by a car battery (DieHard®, Model 30052, Size 51R, Sears, New Orleans, LA) when testing in New Orleans as no electric power was available in the Katrina-affected areas. The sampling flow rate was calibrated with Drycal® DC-Lite Calibrator (Bios International Corp., Butler, NJ) before and after each measurement.
Sampling time needs to be long enough to collect sufficient amount of particulate matter in the submicrometer size range for the subsequent analysis. On the other hand, longer sampling times may lead to particle bounce. During the development of the Fragment Sampling System, it was observed that spore bounce from the collection cup of the second cyclone onto the after-filter can occur if the particle number entering the sampling system exceeds a threshold of 108 particles (Seo et al., 2007). Spores on the after-filter would confound the fragment results as the aim is to collect purified fragments in this particle size fraction. Therefore, the sampling time was chosen based on the overall concentrations of airborne particles as measured by an optical particle counter (OPC; Model 1.108, Grimm Technologies, Inc., Douglasville, GA) operating in parallel with the Fragment Sampling System. The sampling time varied from 120 to 180 minutes.
Traditional fungal spore enumeration was also performed in parallel by collecting aerosol particles onto 25-mm polycarbonate filters (pore size 0.4 μm, GE osmosis Inc., Minnetonka, MN) using the Button Sampler (SKC, Inc., Eighty Four, PA). For reducing pressure drop of this filter, metal screen with a sparse mesh was used as a support pad in the Button Sampler. The Button samplers were attached to battery operated pumps (Model 4004, BGI Inc., Waltham, MA), which produced a flow rate of 41 min−1. The sampling time was the same as for the Fragment Sampling System.
Indoor samples were collected in mold-contaminated rooms during two days. Altogether, three indoor samples were taken in each home during each season. One outdoor sample per day was collected, except in New Orleans during the summer season, because outdoor sampling of fragments could not be performed there at that time due to limitations of the battery power source. Temperature and relative humidity were measured using a traceable humidity/temperature pen (Fisher Scientific Company, Pittsburgh, PA).
2.4 Analytical Methods
The samples collected with the Fragment Sampling System were analyzed for (1→3)-β-D-glucan using the kinetic chromogenic Limulus Amebocyte lysate assay (LAL; Glucatell®, Associates of Cape Cod, East Falmouth, MA) as described before (Seo et al., 2007; Iossifova et al., 2007). Briefly, particles collected into the two cyclones and on the after-filter were extracted into pyrogen-free water (LAL Reagent Water®, Associates of Cape Cod, East Falmouth, MA) containing 0.05% Tween 80. One aliquot of the after-filter suspension was first filtered though a 13-mm mixed cellulose ester (MCE) filter (pore size 1.2 μm, Millipore Corporation, Bedford, MA), which was treated and analyzed for total spore count as described below. The microscopic counting confirmed the absence of spore bounce onto the after-filter. Thus, all extracts were subsequently analyzed by the LAL. Three types of results were calculated from the samples collected with the Fragment Sampling System: (a) total (1→3)-β-D-glucan (all three size fractions combined); (b) (1→3)-β-D-glucan in spore size fraction (>2.25 μm); and (c) (1→3)-β-D-glucan in fragment size fraction (<1.0 μm).
The samples on polycarbonate filter collected by the Button Sampler were extracted similarly as the after-filters, and one aliquot of the extraction suspensions was filtered through a 13-mm MCE filter for microscopic counting. The MCE filters were cleared by acetone vapor and analyzed for total spore count under an optical microscope as described by Adhikari et al. (2003). Spores were identified based on their morphological characteristics. In addition, one aliquot was analyzed for (1→3)-β-D-glucan by the LAL assay to compare (1→3)-β-D-glucan concentrations obtained by the Button Sampler to those obtained by the Fragment Sampling System.
The quality control samples included trip blanks, field blanks, and extraction fluid blanks. The Fragment Sampling System and the Button Samplers were cleaned by first washing with soap, rinsed with water, and finally rinsed with ethanol. All metal parts of cyclone including collection cups and the Button Samplers were treated by heating at 240°C for one hour to remove residual (1→3)-β-D-glucan. The cleaning efficiency was verified by performing LAL-assay for blank samples extracted from a set of cleaned, non-used cyclone collection cups. The quality control samples, analyzed together with the field samples, were all below the limit of detection (2.54 pg ml−1). The median coefficient of variation was 10.3% for the intra-plate variability and 26.0% for the inter-plate variability (Seo et al., 2007).
2.5 Statistical Methods
Data distribution was analyzed by Shapiro Wilk-test. All data were found to be log-normally distributed, except the total (1→3)-β-D-glucan concentration. Thus, geometric means (GM) and geometric standard deviations (GSD) were used for the descriptive statistics. Pearson Correlation Analysis and t-test (or paired t-test) were utilized for the data analysis and calculated from log-transformed data. The statistical analyses were performed using SAS/Stat 9.1 (SAS Institute Inc., Cary, NC), and a significance level of 0.05 was used for all statistical tests.
3. Results
Results on relative humidity and temperature during the environmental sampling are presented in Table 2. The highest values for indoor relative humidity (69.3–90.4%) and temperature (28.9–38.8°C) were measured in New Orleans in the summer. The values were the lowest during the winter measurement cycle.
Table 2.
Relative humidity and temperature during the environmental sampling.
| Relative humidity (%) | Temperature (°C) | ||||
|---|---|---|---|---|---|
| Indoor air | Outdoor air | Indoor air | Outdoor air | ||
| Summer | House 1 | 79.1–89.4 | 32.9–67.2 | 30.0–38.8 | 27.0–33.0 |
| House 2 | 69.3–90.4 | 40.0–53.5 | 28.9–37.3 | 32.1–35.8 | |
| House 3 | 72.8–84.3 | 52.7–71.6 | 30.8–38.1 | 27.8–34.6 | |
| House 4 | 52.4–74.1 | 47.9–67.0 | 15.3–22.0 | 10.0–26.4 | |
| House 5 | 57.0–82.7 | 42.3–59.4 | 12.9–26.2 | 14.8–20.3 | |
|
| |||||
| Winter | House 3 | 48.4–50.8 | 36.0–53.4 | 10.3–17.7 | 12.8–17.3 |
| House 5 | 40.8–48.0 | 40.6–50.0 | 11.6–15.1 | 11.9–14.6 | |
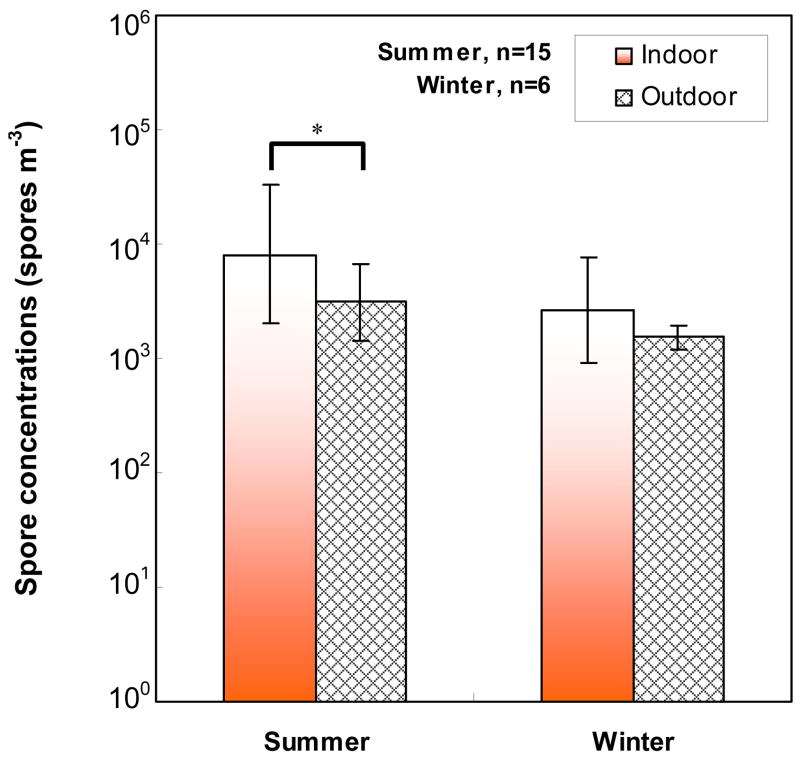
The total fungal spore concentrations as measured with the Button Sampler are shown in Fig. 1 and Table 3. Table 3 shows that the concentrations varied from 0.5 × 103 to 101.1 × 103 spores m−3 in indoor air and from 1.1 × 103 to 8.4 × 103 spores m−3 in outdoor air. The indoor fungal spore concentrations in New Orleans were significantly higher than those in Southern Ohio (P<0.05), whereas the concentrations in outdoor air were similar. The indoor/outdoor (I/O) ratios varied from 0.62 to 9.42 and were significantly higher in New Orleans than in Southern Ohio (P<0.05). The indoor concentrations of fungal spores were slightly higher in the summer than in the winter, but this difference was only borderline significant (P=0.0531). The fungal spore concentrations were higher in indoor air than in outdoor air, but this difference was significant only in the summer (Fig. 1). Aspergillus/Penicillium was the dominant fungal type. In New Orleans, Aspergillus/Penicillium contributed on average of 77.6% to the total spore concentration in the summer and 65.8% in the winter. In Southern Ohio, the corresponding values were 76.9% and 91.7%. The other fungal types detected in indoor samples were the following: Cladosporium, Stachybotrys, Ascospores, Chaetomium Torula, Botrytis, Polythrincium, and Ganoderma.
Fig. 1.
Geometric means, GM, of indoor and outdoor fungal spore concentrations (spores m−3) measured by the Button Sampler. Error bars present geometric standard deviations, GSDs (68% confidence interval), and n is the number of samples. Asterisk and horizontal line present the statistical difference (*: P<0.05).
Table 3.
Indoor and outdoor fungal spore concentrations in New Orleans and Southern Ohio, and indoor airborne (1→3)-β-D-glucan concentration in each size fraction.
| Total fungal spore concentration (spore m−3). (Button Sampler) | Indoor (1→3)-β-D-glucan concentration (pg m−3) (Data obtained with the Fragment Sampling System) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoor | Outdoor | Indoor/Outdoor ratio | Total (1→3)-β-D-glucan | Fragment fraction | Spore fraction | F/S ratio | |||||||
| Summer | |||||||||||||
| New Orleans | 14,942.9 (1,674 – 101,086)
n=9 |
]* | 3,837.0 (1,121 – 8,445)
n=3 |
3.89 (1.35 – 9.42) | 1,460.7 (6,661 – 15,854)
n=9 |
]*** | 192.7 (106 – 87) | ]** | 9,020. 8 (6,080 – 12,894) | ]*** | 0.021 (0.011 – 0.034) | ]* | |
| S. Ohio | 3,234.2 (461 – 10,766)
n=6 |
2,546.3 (1,614 – 3,740)
n=3 |
1.04 (0.79 – 3.61) | 232.5 (192 – 272)
n=6 |
59.6 (18 – 151) | 123.3 (89 – 185) | 0.483 (0.148 – 1.484) | ||||||
|
| |||||||||||||
| Winter | |||||||||||||
| New Orleans | 7,182.8 (5,920 – 8,074)
n=3 |
]** | 1,702.1 (1,346 – 2,513)
n=2 |
4.30 (3.21 – 5.76) | 1,241.1 (707 – 2,097)
n=3 |
520.5 (405 – 652) | 615.8 (251 – 1,358) | 0.845 (0.480 – 1.614) | |||||
| S. Ohio | 940.5 (538 – 1,436)
n=3 |
1,365.7 (1,076 – 1,733)
n=2 |
0.71 (0.62 – 0.82) | 1,128.2 (1,002 – 1,219)
n=3 |
338.0 (104 – 722)
247.0 |
525.0
|
0.644 (0.132 – 2.163) | ||||||
|
| |||||||||||||
| New Orleans alla | 12,442.3 (1,674 – 101,086)
n=12 |
]* | 2,771.9 (1,121 – 8,445)
n=5 |
4.05 (1.35 – 9.42) | ]* | 6,574.5 (707 – 15,854)
n=12 |
]*** | 247.0 (106 – 652) | 4,610.9 (251 – 12,894) | ]*** | 0.054 (0.011 – 1.614) | ]* | |
| S. Ohio all | 2,142.7 (461 – 10,766)
n=9 |
1,984.7 (1,076 – 3,740)
n=5 |
0.90 (0.62 – 3.61) | 393.6 (192 – 1,219)
n=9 |
106.3 (18 – 722) | 199.8 (89 – 792) | 0.532 (0.148 – 2.163) | ||||||
Values present geometric mean (range).
Asterisks present statistical significance (
:P<0.05;
: P<0.01;
P<0.001), and n is the total number of samples.
New Orleans all (or S. Ohio all) include data both in summer and winter.
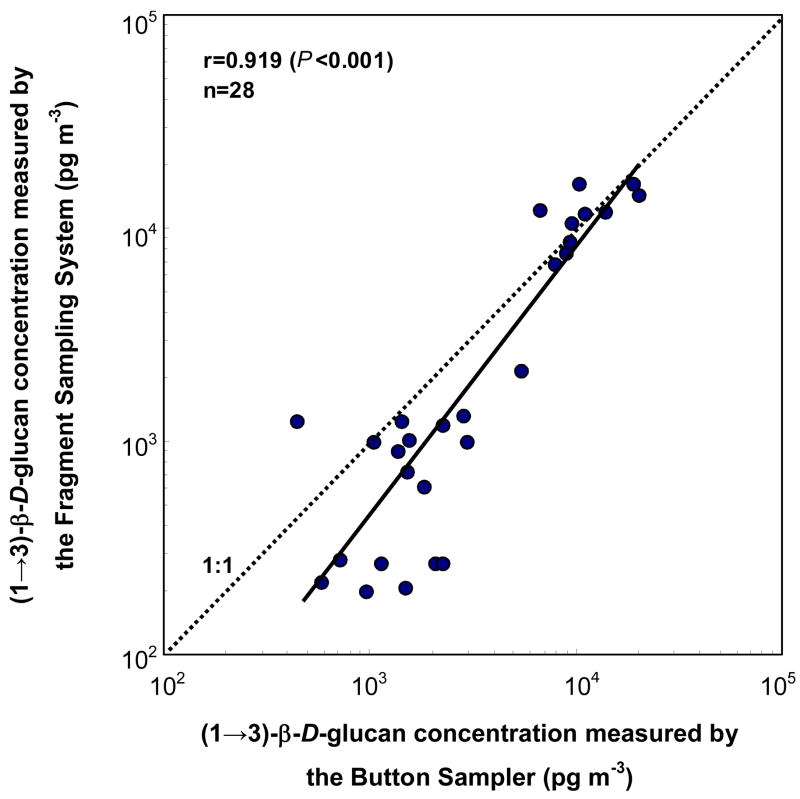
The total (1→3)-β-D-glucan varied from 0.2 to 15.9 ng m−3 and was significantly higher in New Orleans than in Southern Ohio (P<0.001). The total (1→3)-β-D-glucan measured with the Fragment Sampling System correlated well with those measured with the Button Sampler (r=0.919; P<0.001) (Fig. 2). The difference in (1→3)-β-D-glucan measured with these two methods was on average 8.1% and was not significantly different (P=0.066).
Fig. 2.
The correlation between the total (1→3)-β-D-glucan concentrations measured by the Fragment Sampling System and the Button Sampler. Dotted line presents 1:1 ratio; the solid line presents the regression line; and n is the total number of samples.
The concentration of (1→3)-β-D-glucan in the three size fractions ranged from 0.09 to12.9 ng m−3 for spores, from 0.02 to 4.1 ng m−3 for mixture of spores and fragments and from 0.02 to 0.7 ng m−3 for fragments. Similar to the total (1→3)-β-D-glucan, the size-fractioned concentrations in both the fragment and spore size fractions were higher in homes in New Orleans than in homes in Southern Ohio (Table 3). This difference, however, was significant only in the summer (P<0.01 for fragments and P<0.001 for spores).
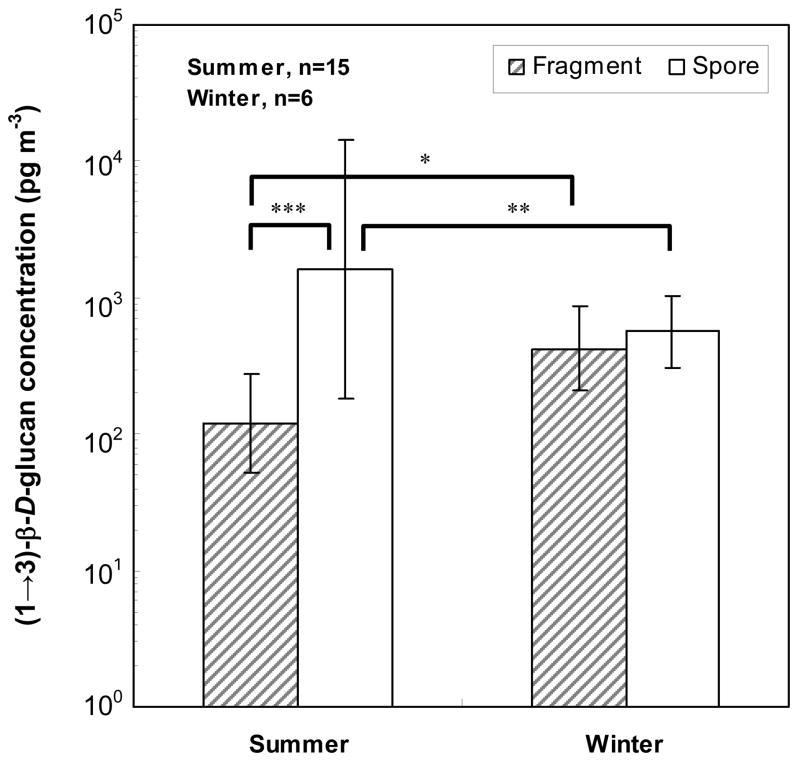
There was a clear seasonal variation in the size-fractionated concentrations: the (1→3)-β-D-glucan in spore size fraction (P<0.01) was higher in the summer than in the winter, whereas the situation was opposite for the (1→3)-β-D-glucan in fragment size fraction (P<0.05) (Fig. 3). In the summer, the (1→3)-β-D-glucan concentration was significantly lower in fragment size fraction than in spore size fraction (P<0.001), but there was no difference in the winter data.
Fig. 3.
Geometric means, GM, of indoor airborne (1→3)-β-D-glucan concentrations (pg m−3) measured by the Fragment Sampling System. Error bars represent geometric standard deviations, GSDs (68% confidence interval), and n is the total number of samples. Asterisk and horizontal line represent the statistical difference (*: P<0.05; **: P<0.01; ***: P<0.001).
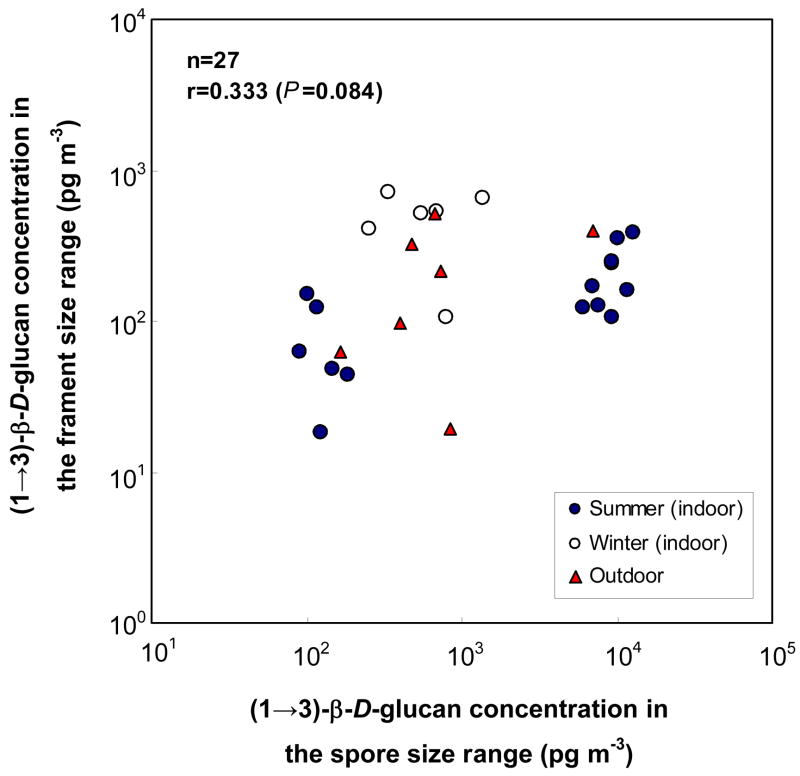
There was only a borderline correlation between the (1→3)-β-D-glucan concentration in the fragment size fraction with that in the spore size fraction when all the data were combined (r=0.333; P=0.084) (Fig. 4). This was driven by the data obtained in the indoor air in the summer as there was a significant correlation only for this data set (r=0.721; P<0.01).
Fig. 4.
Correlation between (1→3)-β-D-glucan concentrations (pg m−3) in fragment and spore size fraction (data obtained by the Fragment Sampling System).
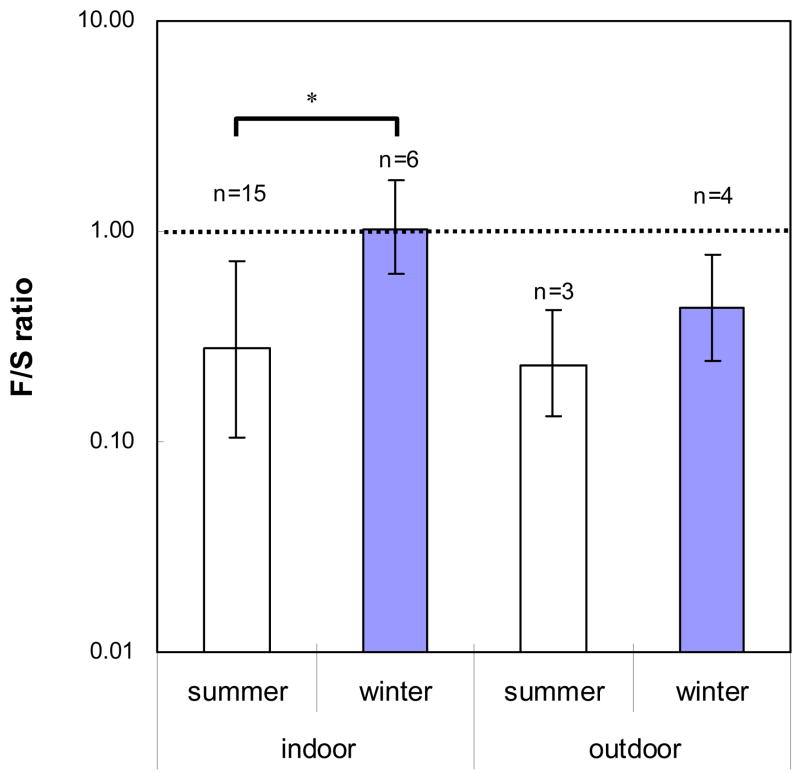
The ratio of (1→3)-β-D-glucan concentration in fragment size fraction to that in spore size fraction (F/S ratio) varied from 0.011 to 2.163 (Table 3). In the summer, the F/S-ratio was significantly lower in New Orleans than in Southern Ohio (P<0.05) but there was no difference in F/S-ratio between these two locations in the winter. The average F/S ratio for the indoor samples collected in the summer (0.277) was significantly lower than for those collected in the winter (1.017) (P <0.05) (Fig. 5).
Fig. 5.
Fragment/spore ratios of (1→3)-β-D-glucan in indoor and outdoor air in the summer and winter (data obtained by the Fragment Sampling System). Histograms represent the arithmetic average and error bars present standard deviations of samples (n). Dotted line presents F/S-ratio=1, and n is total sample numbers. Asterisk and horizontal line represent the statistical difference (*: P<0.05)
4. Discussion
Traditional microbiological methods, such as cultivation and microscopic counting cannot be used for the analysis of fungal fragments. In laboratory-based studies, direct-reading particle counters can be deployed for the enumeration of fragments as non-fungal particles are eliminated in the laboratory setup. In the field situations, fungal fragments are masked by other particles, and a specific assay is needed aiming to analyze fungal components. (1→3)-β-D-glucan was selected as a surrogate of fungal biomass as it is a stable component of fungal cell-wall and does not depend on the viability of the fungi (Stone and Clark, 1992). The recently developed Fragment Sampling System performed well for quantifying the total (1→3)-β-D-glucan when compared side-by-side with a traditional filter-based sampler. This indicates that size-selective sampling and sampling processing caused only minimal sample losses as compared to non-size selective sampling. Furthermore, the microscopic analysis confirmed the absence of spores in fragment samples (on the after-filter).
This study found that the F/S-ratio of (1→3)-β-D-glucan ranged from 0.011 to 2.163, and the highest average, F/S =1.017, was found for the indoor samples collected in the winter. Thus, the (1→3)-β-D-glucan concentration in the fragment size fraction was often equal and in some cases even higher than that in the spore size fraction. The corresponding ratio in a previous laboratory study was 0.029 for fungal particles released from Aspergillus versicolor and 0.034 for Stachybotrys chartarum (Seo et al., 2007). These ratios are based on particle mass as (1→3)-β-D-glucan is analyzed as pg m−3. For number concentration, we have previously shown that the number concentration of fungal fragments was as much as 500 fold higher than that of spores (Cho et al., 2005). In field situations, the number concentration of fragments can be estimated based on the mass of (1→3)-β-D-glucan. Assuming that the mass concentration of fragments and spores is equal (F/S =1) and the spore size is 3.0 μm, the number ratio (fragment number/spore number) would be 103 and 106, if fragment size is 0.3 and 0.03 μm, respectively. These results indicate that the actual (field) contribution of fungal fragments to the overall fungal exposure may be very high, even much higher than it was earlier suggested by our earlier estimates made based on laboratory studies. This can be attributed to the difference in the sampling protocols. In the laboratory tests, fungi were released from the surface directly into the experimental system by high air velocity jets. In the field, the release occurred naturally through variety of mechanisms, including air currents and vibrations, and the release of fragments may have been enhanced by the changes in relative humidity. Furthermore, the larger particles (spores) had time to settle down before sampled from the air.
The clinical significance of fungal fragments is currently not known. However, exposure to fine particles in ambient air has been associated with several adverse health outcomes, including respiratory and cardiac responses (Peters et al., 1997; Von Klot et al., 2002; Penttinen et al., 2001). These health effects have shown stronger associations with the number concentrations of ultrafine particles (<0.1 μm) than with mass or number concentrations of larger particles. Smaller-sized fungal fragments have longer lifetimes in the air compared to spores; they can be easily transported by air currents and more efficiently deposited in the alveolar region than intact spores. Based on laboratory studies on the number concentration and size distribution of fungal particles, it has been estimated that the respiratory deposition of Stachybotrys chartarum fragments in adults is 230 times higher than that of spores (Cho et al., 2005). When the respiratory deposition model was applied to infants, it was demonstrated that the deposition ratios (fragments versus spores) were 4–5 times higher than those for adults (Cho et al., 2005). High particle surface area may further facilitate the bioavailability of fungal components after the particles are inhaled into the human lung. Due to their small size, fragments may be able to evade phagocytosis by macrophages, and can be translocated through systemic circulation (Ibald-Mulli et al., 2002).
(1→3)-β-D-glucan in the spore size fraction appeared to follow similar pattern as the total fungal spore concentration with respect to the seasonal difference and the difference between homes located in New Orleans versus Southern Ohio. However, these patterns were drastically different for (1→3)-β-D-glucan in the fragment size fraction, which did not correlate with the (1→3)-β-D-glucan in the spore size fraction. Highest fragment concentrations were found in the winter, when the fungal spore concentrations were at their lowest levels. This may be attributed to the low relative humidity in the winter, which in turn may increase the release of fungal particles by reducing adhesion forces among fungal structures and enhancing these structures to become brittle. There appears to be few published reports on airborne total fungal spore concentrations determined by the filter-based method. Rao et al. (2007) and Chew et al. (2006) used this method to study New Orleans homes damaged by hurricanes Katrina and Rita within six months after the flooding caused by the hurricanes. Rao et al. (2007) reported geometric mean concentration of 60 × 103 spores m−3 for homes with mild damage (n=5) and 460 × 103 spores m−3 for homes with moderately/heavy damage (n=15). Chew et al. (2006) found total fungal spore concentrations ranging from 82 × 103 to 634 × 103 spores m−3 in three homes before renovation. Osborne et al. (2006) measured total fungal spore concentration in 144 Cincinnati homes that mostly had either no visible mold (44% of homes) or only small area of visible mold (51% of homes had less than 0.2 m2 area of visible mold). The investigators reported a range of 2–2,295 spores m−3 (GM = 145 spores m−3). Lee et al. (2006) studied fungal spore and (1→3)-β-D-glucan concentrations in five non-moldy homes in Cincinnati and found the fungal spore concentration ranging from 105 to 4,961 spores m−3 (GM= 572 spores m−3). The comparison of our data with those published earlier shows that the fungal spore concentrations measured in the present study in New Orleans homes are within the same range as those reported by Chew et al. (2006) and Rao et al. (2007) for the same geographic location. The fungal spore concentrations obtained in Southern Ohio were lower than those measured in New Orleans, which can be explained by the more severe mold damage observed in New Orleans houses. On the other hand, the fungal spore concentrations measured in this study in Southern Ohio homes were somewhat higher than those measured earlier in the same geographic region (city of Cincinnati is located in South-western Ohio) by Lee et al. (2006) and Osborne et al. (2006).
There are no previous reports on (1→3)-β-D-glucan analyzed by the LAL-method in New Orleans, but several investigators have used this method for the analysis of non size-selective air samples in other geographic areas (Thorn and Rylander, 1998; Foto et al., 2005; Lee et al., 2006). Thorn and Rylander (1998) studied 75 homes in Swedish row houses suffering from moisture problems and found the (1→3)-β-D-glucan concentrations up to 19.0 ng m−3. Foto et al. (2005) measured (1→3)-β-D-glucan in 110 Canadian homes, most of which had mold problems. They reported concentrations ranging from 0.04 to 20.55 ng m−3. Lee et al. (2006) reported a range of 0.31–9.35 ng m−3 for five non-moldy homes in Cincinnati. While the indoor (1→3)-β-D-glucan levels measured in New Orleans in this study are much higher than those reported by Lee et al. (2006), they fall to same ranges as reported by Thorn and Rylander (1998) and Foto et al. (2005). The levels measured in Southern Ohio in this study are within the range reported by Lee et al. (2006). These comparisons confirm that the data obtained for New Orleans homes in our study represent well the fungal exposures in non-renovated homes that were affected by flooding in the aftermath of hurricanes Katrina and Rita. On the other hand, the homes in Southern Ohio represent an exposure situation when the concentrations are only slightly or not at all elevated compared to non-moldy homes. This study showed that fungal fragments may represent a significant portion of the fungal exposures in both types of exposure scenarios.
These findings indicate that the actual (field) contribution of fungal fragments to the overall exposure is very high, even much higher than was earlier estimated resulting from the laboratory-generated data. As the exposure to airborne fungal fragments cannot be quantified based on spore concentrations, fragment measurements should be included when assessing exposures in moldy buildings.
Acknowledgments
This work was funded by the NIEHS Center for Environmental Genetics Pilot Project Program (grant #PO30ES006096) and National Institute of Occupational Safety and Health NORA Research Program of the University of Cincinnati Education and Research Center (#T42/CCT510420).
Abbreviations and Acronym list
- F/S-ratio
Fragment/spore-ratio, (1→3)-β-D-glucan concentration in fragment size fraction divided by that in spore size fraction
- GM
Geometric mean
- GSD
Geometric standard deviation
- I/O-ratio
Indoor/outdoor ratio, concentration in indoor air divided by the respective concentration in outdoor air
- LAL
Limulus Amebocyte lysate assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhikari A, Martuzevicius D, Reponen T, Grinshpun SA, Cho SH, Sivasubramani SK, Wei Z, Levin L, Kelley A, Clair GS, LeMasters G. Performance of the Button Personal Inhalable Sampler for the measurement of outdoor aeroallergens. Atmospheric Environment. 2003;34:4723–4733. [Google Scholar]
- Beijer L, Thorn J, Rylander R. Effects after inhalation of (1→3)-beta-D-glucan and relation to mould exposure in the home. Mediators of Inflammation. 2002;11:149–153. doi: 10.1080/09622935020138181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasel TL, Douglas DR, Wilson SC, Straus DC. Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins on paticulates smaller than conidia. Applied and Environmental Microbiology. 2005a;71:114–122. doi: 10.1128/AEM.71.1.114-122.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasel TL, Martin JM, Garriker CG, Wilson SC, Straus DC. Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins in the indoor environment. Applied and Environmental Microbiology. 2005b;71:7376–7388. doi: 10.1128/AEM.71.11.7376-7388.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew GL, Rogers C, Burge HA, Muilenberg ML, Gold DR. Dustborne and airborne fungal particles represent a different spectrum of fungi with differing relations to home characteristics. Allergy. 2003;58:13–20. doi: 10.1034/j.1398-9995.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- Chew GL, Wilson J, Rabito FA, Grimsley F, Iqbal S, Reponen T, Muilenberg ML, Thorne PS, Dearborn DG, Morley RL. Mold and endotoxin levels in the aftermath of hurricane Katrina: a pilot project of homes in New Orleans undergoing renovation. Environmental Health Perspectives. 2006;114:1883–1889. doi: 10.1289/ehp.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Seo SC, Schmechel D, Grinshpun SA, Reponen T. Aerodynamic characteristic and respiratory deposition of fungal particles. Atmospheric Environment. 2005;39:5454–5465. [Google Scholar]
- Foto M, Vrijmoed LLP, Miller JD, Ruest K, Lawton M, Dales RE. A comparison of airborne ergosterol, glucan and Air-O-Cell data in relation to physical assessments of mold damage and some other parameters. Indoor Air. 2005;15:257–266. doi: 10.1111/j.1600-0668.2005.00370.x. [DOI] [PubMed] [Google Scholar]
- Garrett MH, Rayment PR, Hooper MA, Abramson MJ, Hooper BM. Indoor airborne fungal spores, house dampness and associations with environmental factors and respiratory health in children. Clinical & Experimental Allergy. 1998;28:459–467. doi: 10.1046/j.1365-2222.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- Glikson M, Ruthermford S, Simpson RW, Mitchell CA, Yago A. Microscopic and submicron components of atmospheric particulate matter during high asthma periods in Brisbane, Queensland, Australia. Atmospheric Environment. 1995;29:549–562. [Google Scholar]
- Górny RL, Reponen T, Willeke K, Robine E, Boissier M, Grinshpun SA. Fungal fragments as indoor biocontaminants. Applied and Environmental Microbiology. 2002;68:3522–3531. doi: 10.1128/AEM.68.7.3522-3531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górny RL, Mainelis G, Grinshpun SA, Willeke K, Dutkiewicz J, Reponen T. Release of Streptomyces albus propagules from contaminated surfaces. Environmental Research. 2003;91:45–53. doi: 10.1016/s0013-9351(02)00006-3. [DOI] [PubMed] [Google Scholar]
- Green BJ, Sercombe JK, Tovey ER. Fungal fragments and undocumented conidia function as new aeroallergen sources. Journal of Allergy and Clinical Immunology. 2005;115:1043–1048. doi: 10.1016/j.jaci.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Green BJ, Tovey ER, Sercombe JK, Blachere FM, Beezhold DH, Schmechel D. Airborne fungal fragments and allergenicity. Medical Mycology. 2006;44:S245–S255. doi: 10.1080/13693780600776308. [DOI] [PubMed] [Google Scholar]
- Ibald-Mulli A, Wichmann HE, Kreyling W, Peters A. Epidemiological evidence on health effects of ultrafine particles. Journal of Aerosol Medicine. 2002;15:189–201. doi: 10.1089/089426802320282310. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (IOM) Damp Indoor Spaces and Health. The National Academies Press; Washington, D.C. USA: 2004. pp. 65–66. [Google Scholar]
- Iossifova Y, Reponen T, Bernstein D, Levin L, Zeigler H, Kalra H, Campo P, Villareal M, Lockey J, Khurana Hershey GK, LeMasters G. House dust (1→3)-β-D-glucan and wheezing in infants. Allergy. 2007 doi: 10.1111/j.1398-9995.2007.01340.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Grinshpun SA, Kim KY, Iossifova Y, Adhikari A, Reponen T. Relationship between indoor and outdoor airborne fungal spores, pollen, and (1→3)-β-D-glucan in homes without visible mold growth. Aerobiologia. 2006;22:227–236. [Google Scholar]
- Li DW, Kendrick B. A year-round comparison of fungal spores in indoor and outdoor airborne fungi. Canadian Journal of Botany. 1995;74:194–209. [Google Scholar]
- Nevalainen A, Pasanen AL, Niininen M, Reponen T, Jantunen MJ, Kalliokoski P. The indoor air quality in Finnish homes with mold problems. Environment International. 1991;17:299–302. [Google Scholar]
- Osborne M, Reponen T, Adhikari A, Cho SH, Grinshpun SA, Levin L, Bernstein DI, LeMasters G. Specific fungal exposures, allergic sensitization, and rhinitis in infants. Pediatric Allergy and Immunology. 2006;17:450–457. doi: 10.1111/j.1399-3038.2006.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttinen P, Timonen KL, Tiittanen P, Mirme A, Ruuskanen J, Pekkanen J. Ultrafine particles in urban air and respiratory health among adult asthmatics. European Respiratory Journal. 2001;17:428–435. doi: 10.1183/09031936.01.17304280. [DOI] [PubMed] [Google Scholar]
- Peters A, Wichmann HE, Tuch T, Heinrich J, Heyder J. Respiratory effects are associated with the number of ultrafine particles. American Journal of Respiratory and Critical Care Medicine. 1997;155:1376–1383. doi: 10.1164/ajrccm.155.4.9105082. [DOI] [PubMed] [Google Scholar]
- Rao CY, Riggs MA, Chew GL, Muilenberg ML, Thorne PS, Van Sickle D, Dunn KH, Clive Brown C. Characterization of airborne molds, endotoxins, and glucans in homes in New Orleans after Hurricanes Katrina and Rita. Applied and Environmental Microbiology. 2007;73:1630–1634. doi: 10.1128/AEM.01973-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SC, Grinshpun SA, Iossifova Y, Schmechel D, Rao C, Reponen T. A new field-compatible methodology for the collection and analysis of fungal fragments. Aerosol Science and Technology. 2007 in press. [Google Scholar]
- Stone BA, Clarke AE. The chemistry and biology of (1-3)-β-glucan. La Trobe University Press; 1992. pp. 283–363. [Google Scholar]
- Strachan DP, Flannigan B, McCabe EM, McGarry F. Quantification of airborne moulds in the homes of children with and without wheeze. Thorax. 1990;45:382–387. doi: 10.1136/thx.45.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn J, Rylander R. Airways inflammation and glucan in damp row-houses. American Journal of Respiratory and Critical Care Medicine. 1998;157:1798–1803. doi: 10.1164/ajrccm.157.6.9706081. [DOI] [PubMed] [Google Scholar]
- Von Klot S, Wolke G, Tuch T, Heinrich J, Dockery DW, Schwartz J, Kreyling WG, Wichmann HE, Peters A. Increased asthma medication use in association with ambient fine and ultrafine particles. European Respiratory Journal. 2002;20:691–702. doi: 10.1183/09031936.02.01402001. [DOI] [PubMed] [Google Scholar]