Abstract
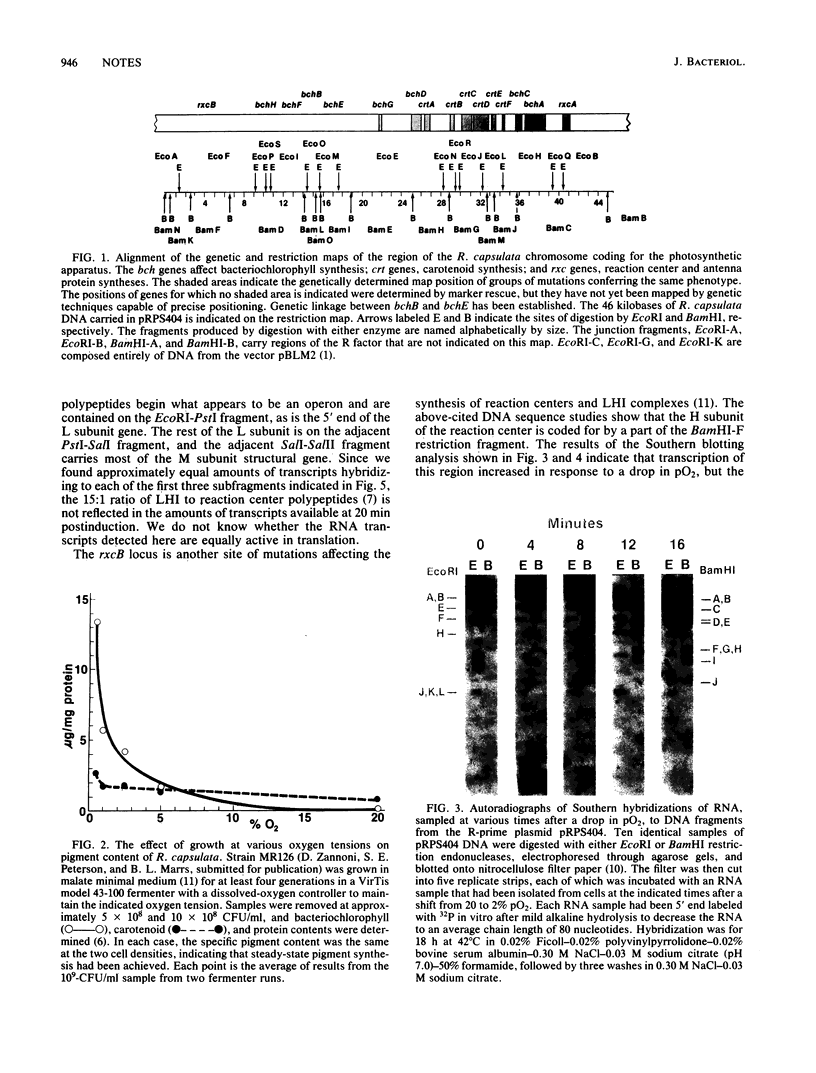
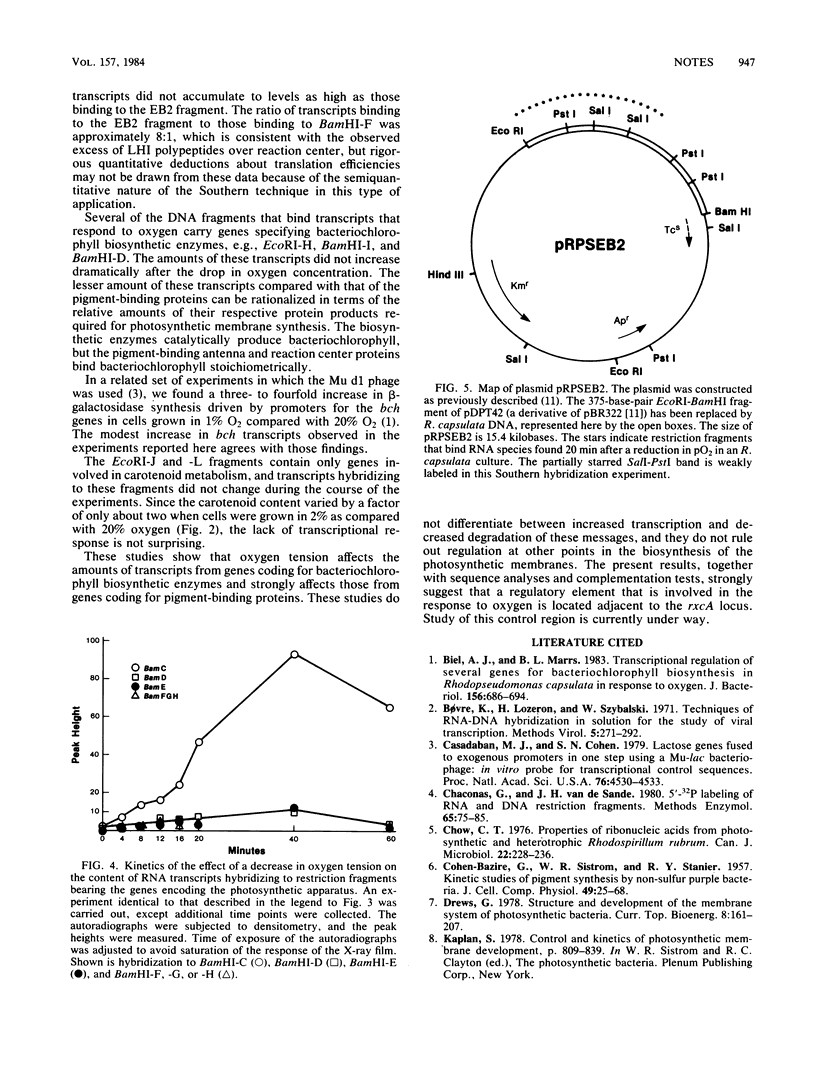
The effect of oxygen tension on the transcription of genes coding for the photosynthetic apparatus of Rhodopseudomonas capsulata was determined by the Southern hybridization technique. Restriction endonuclease digests of the R-prime plasmid pRPS404 and a subcloned fragment thereof served as DNA probes for genetically defined regions. The results showed that transcripts corresponding to the genes for certain pigment-binding polypeptides increase in amount by about 40-fold after a drop in oxygen tension. Transcripts hybridizing to genes involved in bacteriochlorophyll biosynthesis increase to a much lesser extent, and several genes involved in carotenoid biosynthesis are not affected by pO2.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biel A. J., Marrs B. L. Transcriptional regulation of several genes for bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata in response to oxygen. J Bacteriol. 1983 Nov;156(2):686–694. doi: 10.1128/jb.156.2.686-694.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaconas G., van de Sande J. H. 5'-32P labeling of RNA and DNA restriction fragments. Methods Enzymol. 1980;65(1):75–85. doi: 10.1016/s0076-6879(80)65012-5. [DOI] [PubMed] [Google Scholar]
- Chow C. T. Properties of ribonucleic acids from photosynthetic and heterotrophic Rhodospirillum rubrum. Can J Microbiol. 1976 Feb;22(2):228–236. doi: 10.1139/m76-031. [DOI] [PubMed] [Google Scholar]
- Marrs B. Mobilization of the genes for photosynthesis from Rhodopseudomonas capsulata by a promiscuous plasmid. J Bacteriol. 1981 Jun;146(3):1003–1012. doi: 10.1128/jb.146.3.1003-1012.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N., Clark W. G., Marrs B. L. Alignment of genetic and restriction maps of the photosynthesis region of the Rhodopseudomonas capsulata chromosome by a conjugation-mediated marker rescue technique. J Bacteriol. 1983 May;154(2):580–590. doi: 10.1128/jb.154.2.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver P. F., Wall J. D., Gest H. Characterization of Rhodopseudomonas capsulata. Arch Microbiol. 1975 Nov 7;105(3):207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]
- Witkin S. S., Gibson K. D. Ribonucleic acid from aerobically and anaerobically grown Rhodopseudomonas spheroides: comparison by hybridization to chromosomal and satellite deoxyribonucleic acid. J Bacteriol. 1972 May;110(2):684–690. doi: 10.1128/jb.110.2.684-690.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]




