Abstract
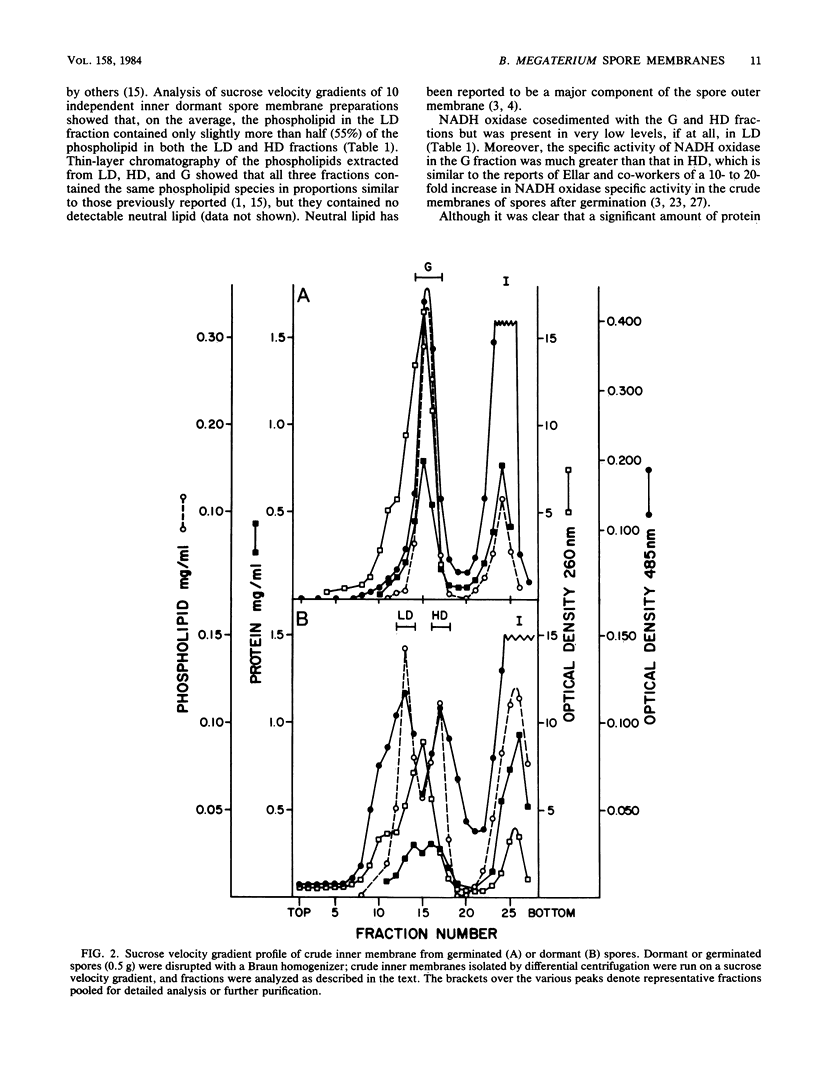
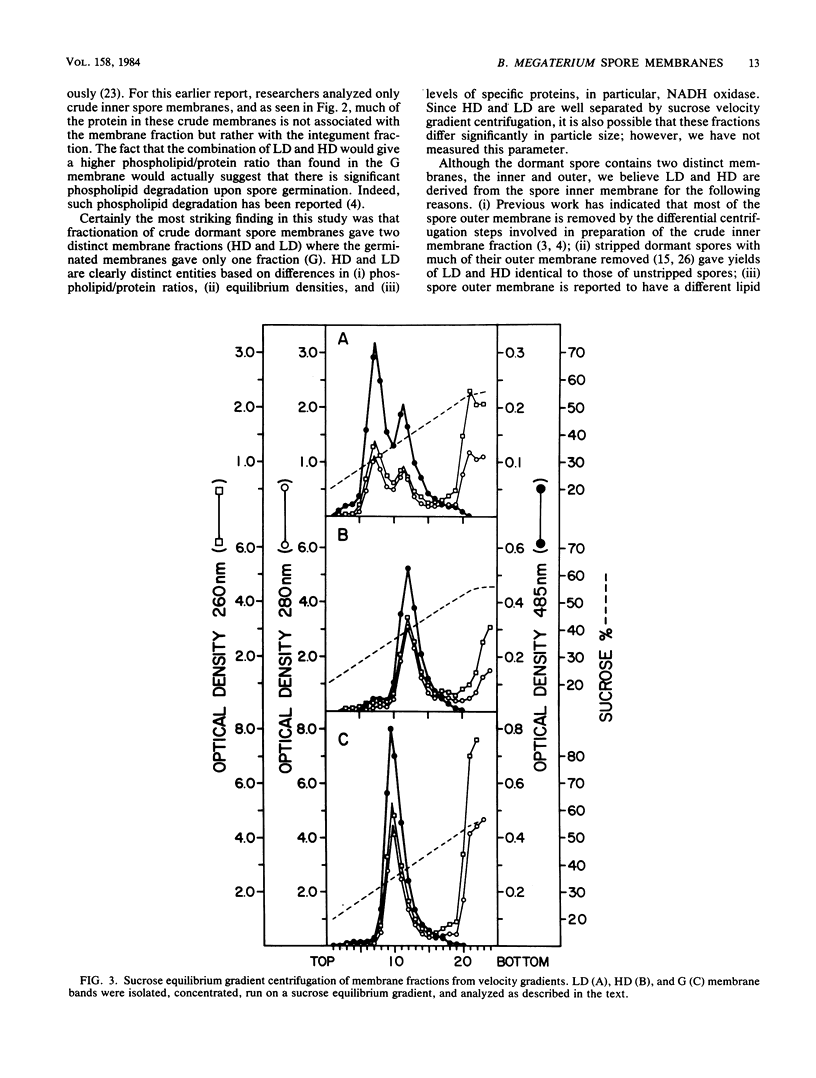
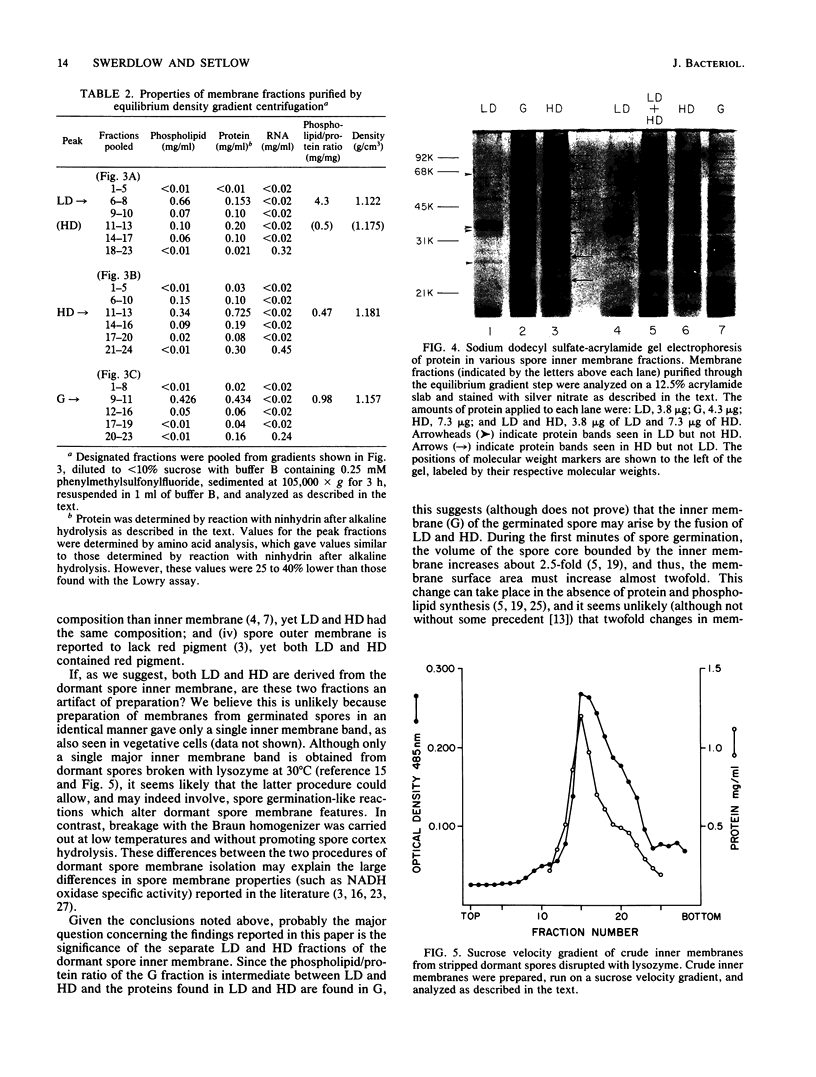
Two distinct membrane bands were obtained after sucrose velocity gradient centrifugation of crude inner membranes from dormant Bacillus megaterium spores disrupted under conditions which minimized endogenous enzyme action. These two inner membrane fractions (termed LD and HD) contained similar amounts of total and individual phospholipid species. However, LD and HD differed significantly in phospholipid/protein ratios (4.3 and 0.47 mg/mg, respectively), equilibrium densities (1.12 and 1.18 g/cm3), NADH oxidase specific activity (less than 0.01 and 0.13 mumol/min X mg), and content of specific proteins. In contrast, crude membranes prepared in identical fashion from germinated spores gave only a single inner membrane band (termed G) on sucrose velocity gradients. G had a phospholipid/protein ratio of 0.98 mg/mg, an equilibrium density of 1.16 g/cm3, and an NADH oxidase specific activity of 2.1 mumol/min X mg. Essentially all of the proteins present in LD or HD or both were found in G, consistent with the latter membrane being derived from a mixture of LD and HD. No evidence was found suggesting that there is significant degradation of dormant spore inner membrane protein upon spore germination.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertsch L. L., Bonsen P. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XIV. Phospholipids in Bacillus megaterium. J Bacteriol. 1969 Apr;98(1):75–81. doi: 10.1128/jb.98.1.75-81.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Lighty A., Ellar D. J. The structure and function of the spore outer membrane in dormant and germinating spores of Bacillus megaterium. J Appl Bacteriol. 1980 Feb;48(1):135–145. doi: 10.1111/j.1365-2672.1980.tb05215.x. [DOI] [PubMed] [Google Scholar]
- Guinand M., Vacheron M. J., Michel G., Tipper D. J. Location of peptidoglycan lytic enzymes in Bacillus sphaericus. J Bacteriol. 1979 Apr;138(1):126–132. doi: 10.1128/jb.138.1.126-132.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A. S., Coughlin R. T., Racine F. M., McGroarty E. J., Vary J. C. Use of electron spin resonance to study Bacillus megaterium spore membranes. Biochem Biophys Res Commun. 1979 Jul 27;89(2):565–570. doi: 10.1016/0006-291x(79)90667-3. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Tipper D. J. Acid-soluble spore proteins of Bacillus subtilis. J Bacteriol. 1981 Jun;146(3):972–982. doi: 10.1128/jb.146.3.972-982.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncewicz M. A., Ellar D. J., Posgate J. A. Metabolism of membrane lipids during bacterial spore germination and outgrowth. Biochem Soc Trans. 1977;5(1):118–119. doi: 10.1042/bst0050118. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Norisuye T., Yu H. Osmotically-induced and photo-induced deformations of disk membranes. Biochim Biophys Acta. 1977 Dec 15;471(3):436–452. doi: 10.1016/0005-2736(77)90048-7. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Racine F. M., Vary J. C. Improved method for purification of membranes from spores of Bacillus Megaterium. Prep Biochem. 1982;12(3):265–273. doi: 10.1080/00327488208065567. [DOI] [PubMed] [Google Scholar]
- Racine F. M., Vary J. C. Isolation and properties of membranes from Bacillus megaterium spores. J Bacteriol. 1980 Sep;143(3):1208–1214. doi: 10.1128/jb.143.3.1208-1214.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Kennedy E. P. Asymmetrical distribution of phospholipids in the membrane of Bacillus megaterium. J Mol Biol. 1977 Mar 5;110(3):603–618. doi: 10.1016/s0022-2836(77)80114-9. [DOI] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Measurements of the pH within dormant and germinated bacterial spores. Proc Natl Acad Sci U S A. 1980 May;77(5):2474–2476. doi: 10.1073/pnas.77.5.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XVII. Sulfhydryl and disulfide levels in dormancy and germination. J Bacteriol. 1969 Dec;100(3):1155–1160. doi: 10.1128/jb.100.3.1155-1160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Ozols J. The complete covalent structure of protein B. The third major protein degraded during germination of Bacillus megaterium spores. J Biol Chem. 1980 Nov 10;255(21):10445–10450. [PubMed] [Google Scholar]
- Seto-Young D. L., Ellar D. J. Membrane changes during germination of Bacillus megaterium KM spores. Microbios. 1979;26(103):7–15. [PubMed] [Google Scholar]
- Stewart G. S., Eaton M. W., Johnstone K., Barrett M. D., Ellar D. J. An investigation of membrane fluidity changes during sporulation and germination of Bacillus megaterium K.M. measured by electron spin and nuclear magnetic resonance spectroscopy. Biochim Biophys Acta. 1980 Aug 4;600(2):270–290. doi: 10.1016/0005-2736(80)90432-0. [DOI] [PubMed] [Google Scholar]
- Swerdlow B. M., Setlow B., Setlow P. Levels of H+ and other monovalent cations in dormant and germinating spores of Bacillus megaterium. J Bacteriol. 1981 Oct;148(1):20–29. doi: 10.1128/jb.148.1.20-29.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary J. C. Germination of Bacillus megaterium spores after various extraction procedures. J Bacteriol. 1973 Nov;116(2):797–802. doi: 10.1128/jb.116.2.797-802.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Ellar D. J., Scott I. R., Koncewicz M. A. Rapid, chloramphenicol-resistant, activation of membrane electron transport on germination of Bacillus spores. Nature. 1977 Mar 10;266(5598):174–176. doi: 10.1038/266174a0. [DOI] [PubMed] [Google Scholar]