Abstract
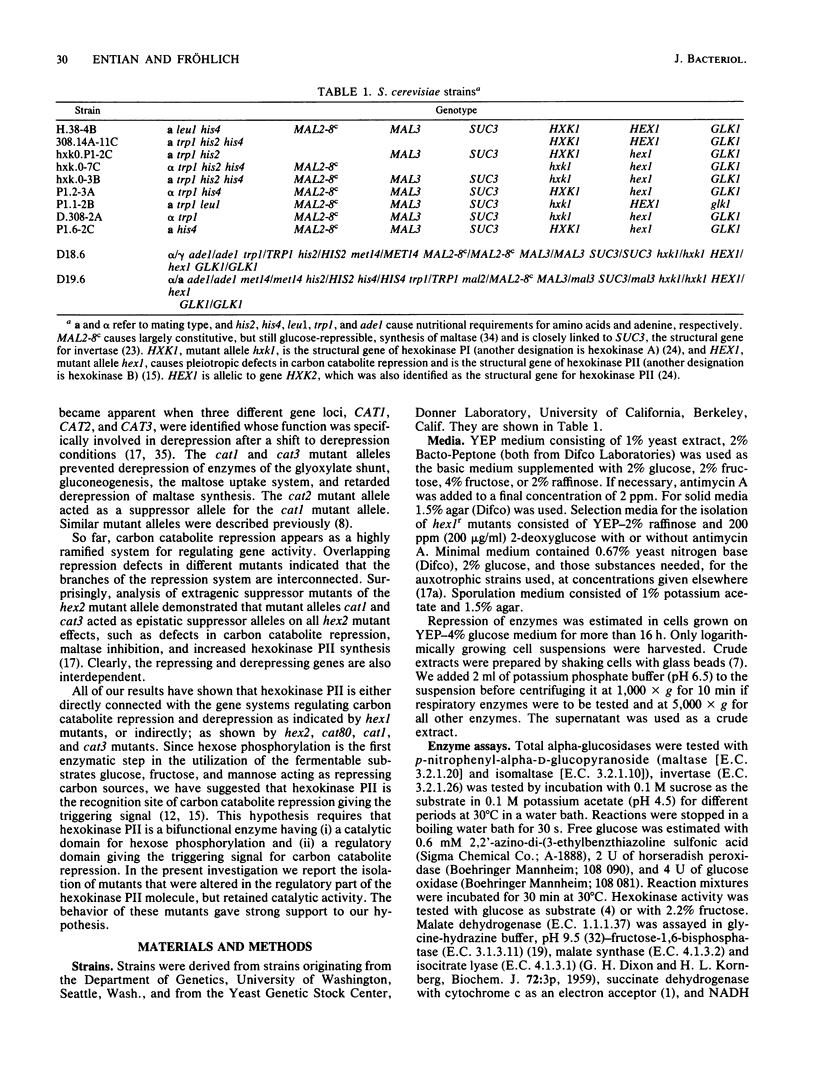
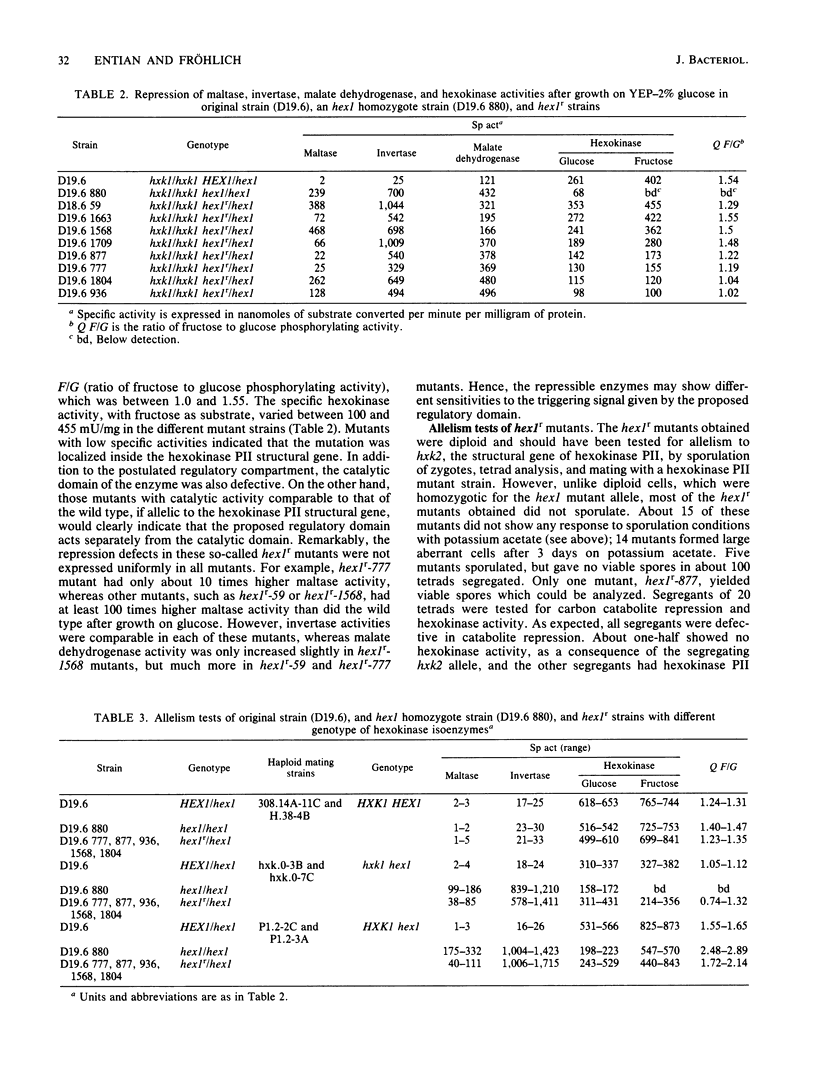
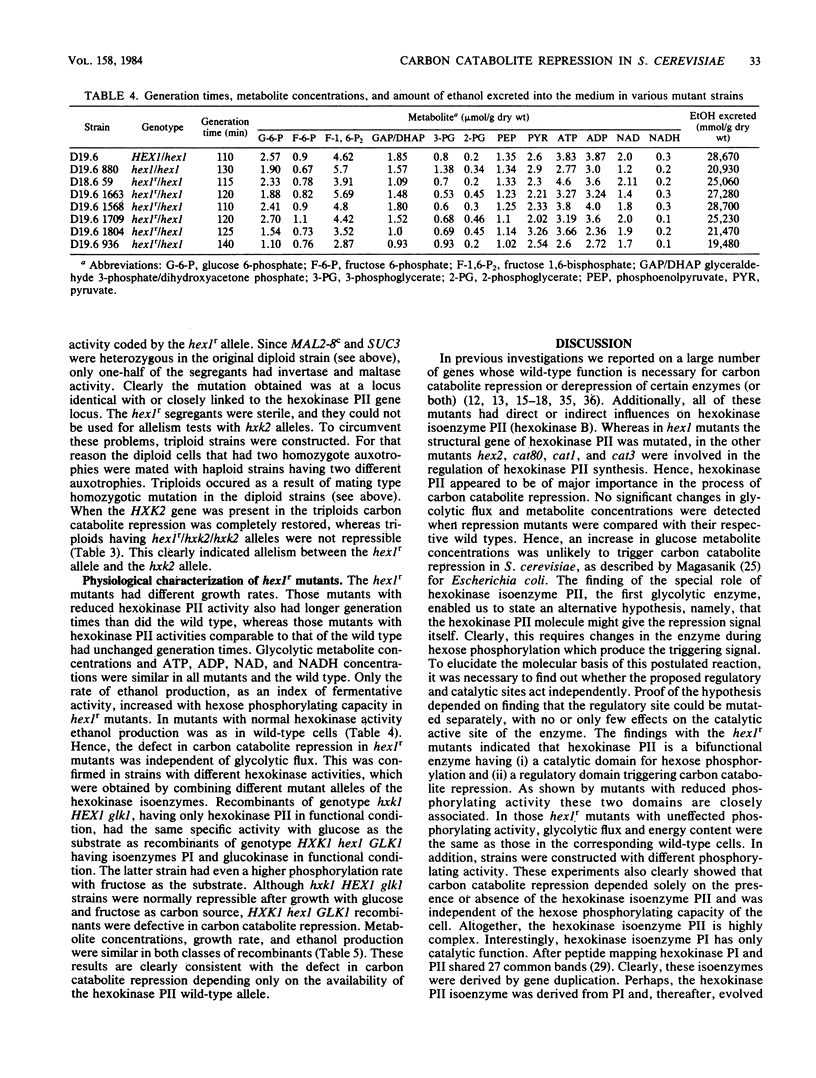
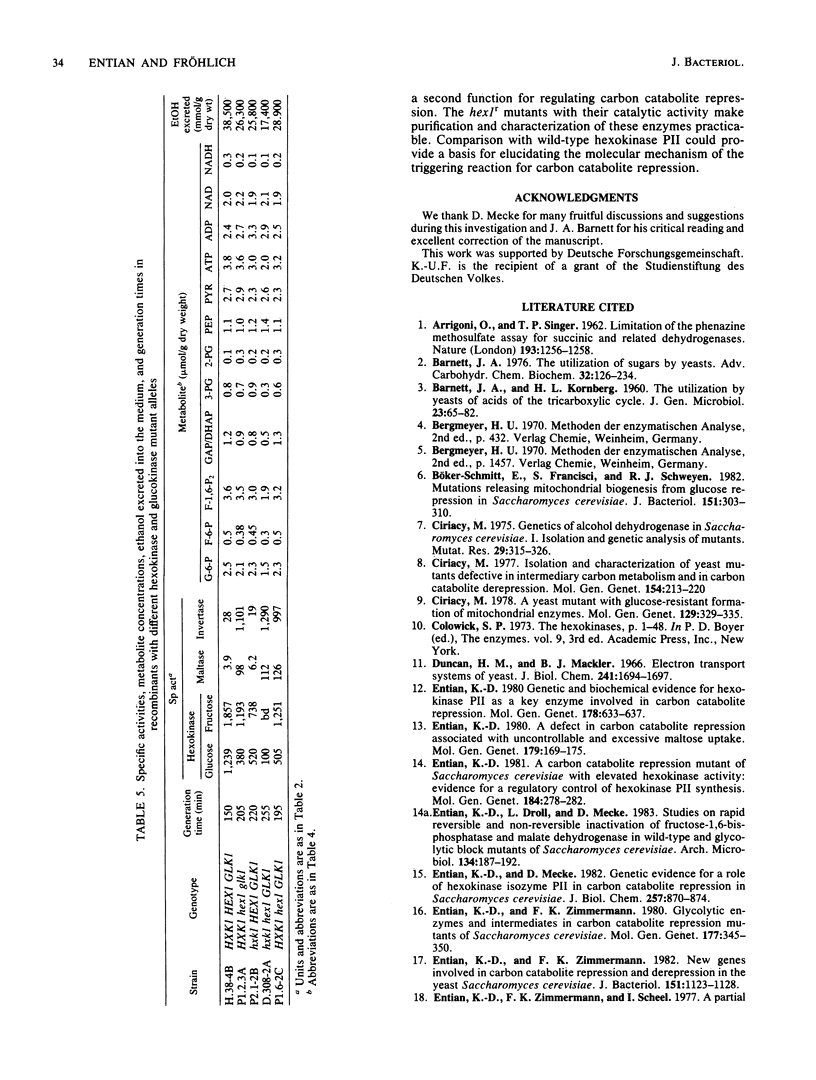
A selection system has been devised for isolating hexokinase PII structural gene mutants that cause defects in carbon catabolite repression, but retain normal catalytic activity. We used diploid parental strains with homozygotic defects in the hexokinase PI structural gene and with only one functional hexokinase PII allele. Of 3,000 colonies tested, 35 mutants (hex1r) did not repress the synthesis of invertase, maltase, malate dehydrogenase, and respiratory enzymes. These mutants had additional hexokinase PII activity. In contrast to hex1 mutants (Entian et al., Mol. Gen. Genet. 156:99-105, 1977; F.K. Zimmermann and I. Scheel, Mol. Gen. Genet. 154:75-82, 1977), which were allelic to structural gene mutants of hexokinase PII and had no catalytic activity (K.-D. Entian, Mol. Gen. Gent. 178:633-637, 1980), the hex1r mutants sporulated hardly at all or formed aberrant cells. Those ascospores obtained were mostly inviable. As the few viable hex1r segregants were sterile, triploid cells were constructed to demonstrate allelism between hex1r mutants and hexokinase PII structural gene mutants. Metabolite concentrations, growth rate, and ethanol production were the same in hex1r mutants and their corresponding wild-type strains. Recombination of hexokinase and glucokinase alleles gave strains with different specific activities. The defect in carbon catabolite repression was strongly associated with the defect in hexokinase PII and was independent of the glucose phosphorylating capacity. Hence, a secondary effect caused by reduced hexose phosphorylation was not responsible for the repression defect in hex1 mutants. These results, and those with the hex1r mutants isolated, strongly supported our earlier hypothesis that hexokinase PII is a bifunctional enzyme with (i) catalytic activity and (ii) a regulatory component triggering carbon catabolite repression (Entian, Mol. Gen. Genet. 178:633-637, 1980; K.-D. Entian and D. Mecke, J. Biol. Chem. 257:870-874, 1982).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- BARNETT J. A., KORNBERG H. L. The utilization by yeasts of acids of the tricarboxylic acid cycle. J Gen Microbiol. 1960 Aug;23:65–82. doi: 10.1099/00221287-23-1-65. [DOI] [PubMed] [Google Scholar]
- Barnett J. A. The utilization of sugars by yeasts. Adv Carbohydr Chem Biochem. 1976;32:125–234. doi: 10.1016/s0065-2318(08)60337-6. [DOI] [PubMed] [Google Scholar]
- Böker-Schmitt E., Francisci S., Schweyen R. J. Mutations releasing mitochondrial biogenesis from glucose repression in Saccharomyces cerevisiae. J Bacteriol. 1982 Jul;151(1):303–310. doi: 10.1128/jb.151.1.303-310.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriacy M. A yeast mutant with glucose-resistant formation of mitochondrial enzymes. Mol Gen Genet. 1978 Feb 27;159(3):329–335. doi: 10.1007/BF00268270. [DOI] [PubMed] [Google Scholar]
- Ciriacy M. Isolation and characterization of yeast mutants defective in intermediary carbon metabolism and in carbon catabolite derepression. Mol Gen Genet. 1977 Jul 20;154(2):213–220. doi: 10.1007/BF00330840. [DOI] [PubMed] [Google Scholar]
- Duncan H. M., Mackler B. Electron transport systems of yeast. 3. Preparation and properties of cytochrome oxidase. J Biol Chem. 1966 Apr 25;241(8):1694–1697. [PubMed] [Google Scholar]
- Entian K. D. A carbon catabolite repression mutant of Saccharomyces cerevisiae with elevated hexokinase activity: evidence for regulatory control of hexokinase PII synthesis. Mol Gen Genet. 1981;184(2):278–282. doi: 10.1007/BF00272917. [DOI] [PubMed] [Google Scholar]
- Entian K. D. A defect in carbon catabolite repression associated with uncontrollable and excessive maltose uptake. Mol Gen Genet. 1980;179(1):169–175. doi: 10.1007/BF00268460. [DOI] [PubMed] [Google Scholar]
- Entian K. D., Dröll L., Mecke D. Studies on rapid reversible and non-reversible inactivation of fructose-1,6-bisphosphatase and malate dehydrogenase in wild-type and glycolytic block mutants of Saccharomyces cerevisiae. Arch Microbiol. 1983 Jun;134(3):187–192. doi: 10.1007/BF00407756. [DOI] [PubMed] [Google Scholar]
- Entian K. D. Genetic and biochemical evidence for hexokinase PII as a key enzyme involved in carbon catabolite repression in yeast. Mol Gen Genet. 1980;178(3):633–637. doi: 10.1007/BF00337871. [DOI] [PubMed] [Google Scholar]
- Entian K. D., Mecke D. Genetic evidence for a role of hexokinase isozyme PII in carbon catabolite repression in Saccharomyces cerevisiae. J Biol Chem. 1982 Jan 25;257(2):870–874. [PubMed] [Google Scholar]
- Entian K. D., Zimmermann F. K. Glycolytic enzymes and intermediates in carbon catabolite repression mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1980 Jan;177(2):345–350. doi: 10.1007/BF00267449. [DOI] [PubMed] [Google Scholar]
- Entian K. D., Zimmermann F. K. New genes involved in carbon catabolite repression and derepression in the yeast Saccharomyces cerevisiae. J Bacteriol. 1982 Sep;151(3):1123–1128. doi: 10.1128/jb.151.3.1123-1128.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich K. U., Entian K. D., Mecke D. Cloning and restriction analysis of the hexokinase PII gene of the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1984;194(1-2):144–148. doi: 10.1007/BF00383509. [DOI] [PubMed] [Google Scholar]
- GANCEDO C., SALAS M. L., GINER A., SOLS A. RECIPROCAL EFFECTS OF CARBON SOURCES ON THE LEVELS OF AN AMP-SENSITIVE FRUCTOSE-1,6-DIPHOSPHATASE AND PHOSPHOFRUCTOKINASE IN YEAST. Biochem Biophys Res Commun. 1965 Jun 18;20:15–20. doi: 10.1016/0006-291x(65)90944-7. [DOI] [PubMed] [Google Scholar]
- Gancedo C., Schwerzmann K. Inactivation by glucose of phosphoenolpyruvate carboxykinase from Saccharomyces cerevisiae. Arch Microbiol. 1976 Sep 1;109(3):221–225. doi: 10.1007/BF00446632. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M., Gancedo C. Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non fermenting yeasts. Arch Mikrobiol. 1971;76(2):132–138. doi: 10.1007/BF00411787. [DOI] [PubMed] [Google Scholar]
- Gascón S., Neumann N. P., Lampen J. O. Comparative study of the properties of the purified internal and external invertases from yeast. J Biol Chem. 1968 Apr 10;243(7):1573–1577. [PubMed] [Google Scholar]
- Grossmann M. K., Zimmermann F. K. The structural genes of internal invertases in Saccharomyces cerevisiae. Mol Gen Genet. 1979 Sep;175(2):223–229. doi: 10.1007/BF00425540. [DOI] [PubMed] [Google Scholar]
- Lobo Z., Maitra P. K. Genetics of yeast hexokinase. Genetics. 1977 Aug;86(4):727–744. doi: 10.1093/genetics/86.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem J. 1965 Oct;97(1):284–297. doi: 10.1042/bj0970284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W., Meek G. A. Changes in the activities of respiratory enzymes during the aerobic growth of yeast on different carbon sources. Biochem J. 1965 Oct;97(1):298–302. doi: 10.1042/bj0970298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. J., Colowick S. P. Chemistry and subunit structure of yeast hexokinase isoenzymes. Arch Biochem Biophys. 1973 Oct;158(2):458–470. doi: 10.1016/0003-9861(73)90537-7. [DOI] [PubMed] [Google Scholar]
- Van Wijk R., Ouwehand J., van den Bos T., Koningsberger V. V. Induction and catabolite repression of alpha-glucosidase synthesis in protoplasts of Saccharomyces carlsbergensis. Biochim Biophys Acta. 1969 Jul 22;186(1):178–191. doi: 10.1016/0005-2787(69)90501-2. [DOI] [PubMed] [Google Scholar]
- WOLFE R. G., NEILANDS J. B. Some molecular and kinetic properties of heart malic dehydrogenase. J Biol Chem. 1956 Jul;221(1):61–69. [PubMed] [Google Scholar]
- Witt I., Kronau R., Holzer H. Repression von Alkoholdehydrogenase, Malatdehydrogenase, Isocitratlyase und Malatsynthase in Hefe durch Glucose. Biochim Biophys Acta. 1966 Jun 15;118(3):522–537. [PubMed] [Google Scholar]
- Zimmermann F. K., Eaton N. R. Genetics of induction and catabolite repression of Maltese synthesis in Saccharomyces cerevisiae. Mol Gen Genet. 1974;134(3):261–272. doi: 10.1007/BF00267720. [DOI] [PubMed] [Google Scholar]
- Zimmermann F. K., Kaufmann I., Rasenberger H., Haubetamann P. Genetics of carbon catabolite repression in Saccharomycess cerevisiae: genes involved in the derepression process. Mol Gen Genet. 1977 Feb 28;151(1):95–103. doi: 10.1007/BF00446918. [DOI] [PubMed] [Google Scholar]
- Zimmermann F. K., Scheel I. Mutants of Saccharomyces cerevisiae resistant to carbon catabolite repression. Mol Gen Genet. 1977 Jul 7;154(1):75–82. doi: 10.1007/BF00265579. [DOI] [PubMed] [Google Scholar]



