Abstract
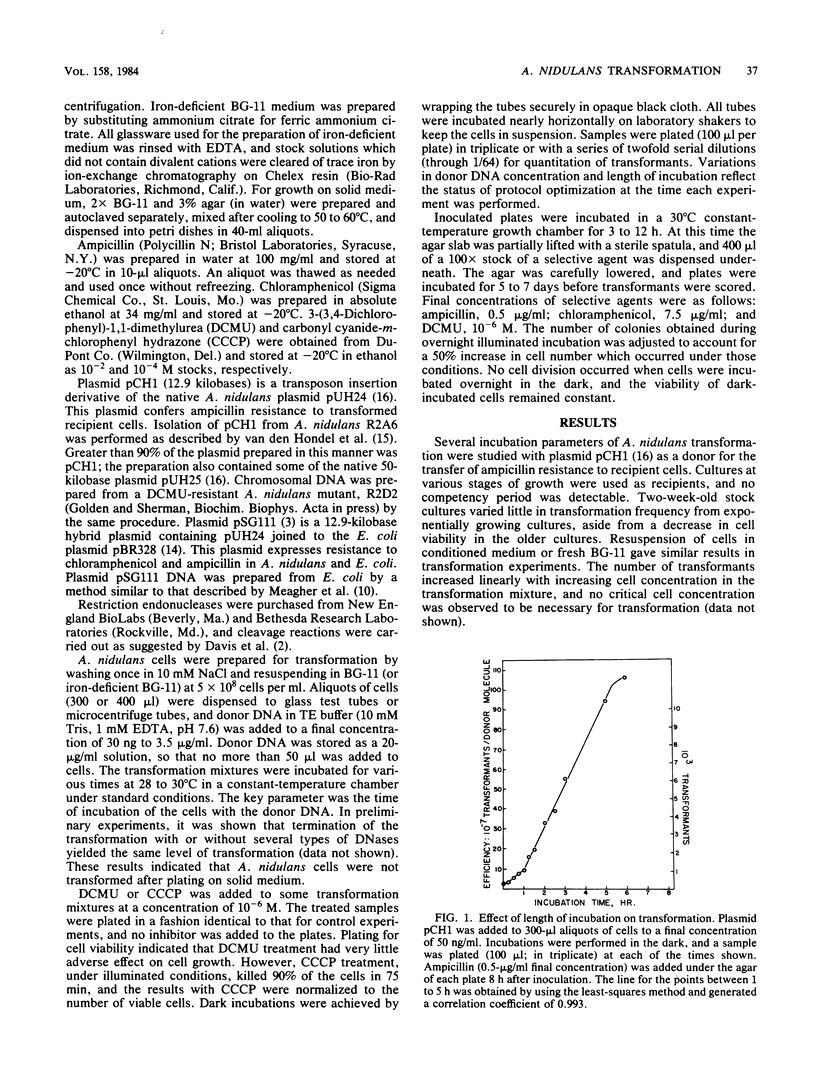
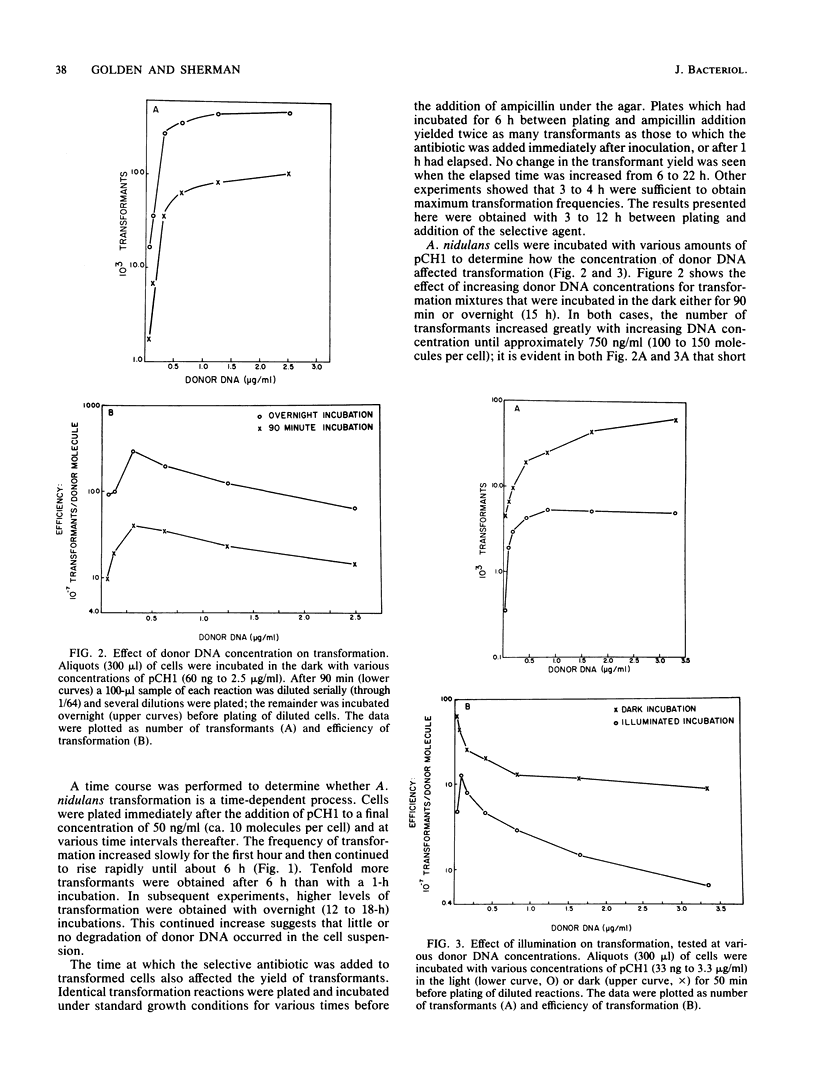
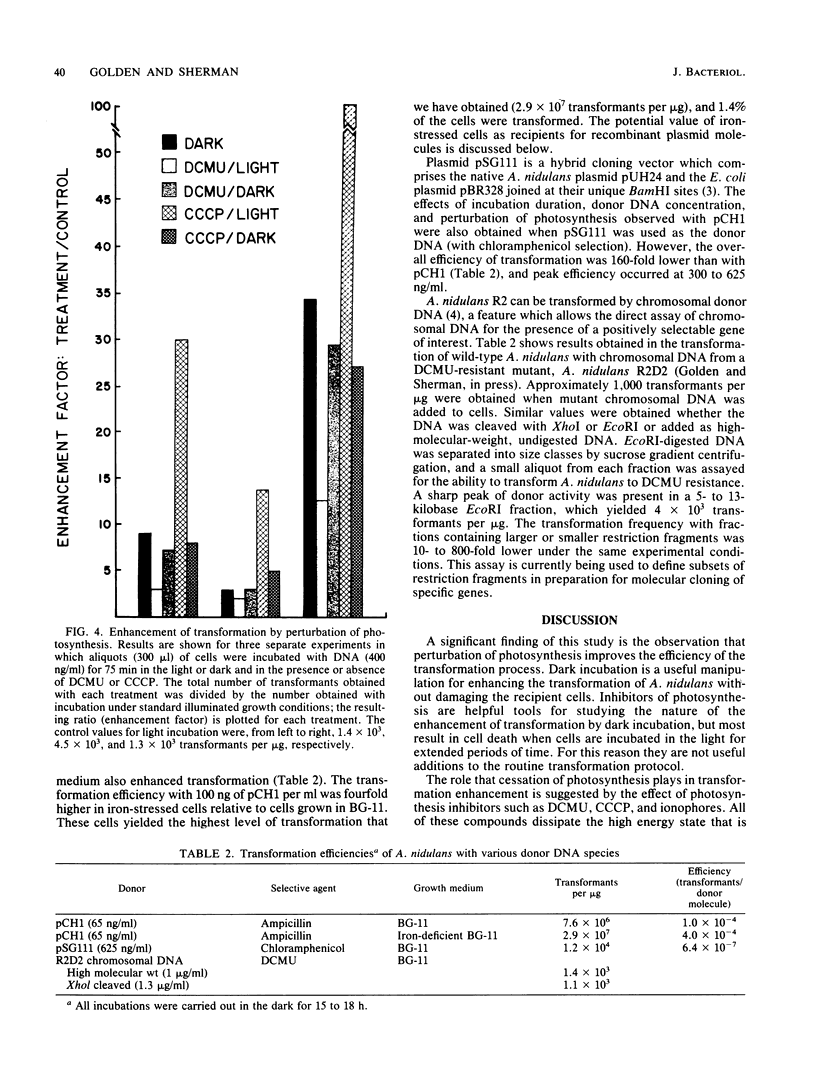
Under optimal conditions, the cyanobacterium Anacystis nidulans R2 was transformed to ampicillin resistance at frequencies of greater than 10(7) transformants per microgram of plasmid (pCH1) donor DNA. No stringent period of competency was detected, and high frequencies of transformation were achieved with cultures at various growth stages. Transformation increased with time after addition of donor DNA up to 15 to 18 h. The peak of transformation efficiency (transformants/donor molecule) occurred at plasmid concentrations of 125 to 325 ng/ml with an ampicillin resistance donor plasmid (pCH1) and 300 to 625 ng/ml for chloramphenicol resistance conferred by plasmid pSG111. The efficiency of transformation was enhanced by excluding light during the incubation or by blocking photosynthesis with the electron transport inhibitor 3-(3, 4-dichlorophenyl)-1, 1-dimethylurea (DCMU) or the uncoupler carbonyl cyanide-m-chlorophenyl hydrazone. Preincubation of cells in darkness for 15 to 18 h before addition of donor DNA significantly decreased transformation efficiency. Growth of cells in iron-deficient medium before transformation enhanced efficiency fourfold. These results were obtained with selection for ampicillin (pCH1 donor plasmid)- or chloramphenicol (pSG111 donor plasmid)-resistant transformants. Approximately 1,000 transformants per microgram were obtained when chromosomal DNA from an herbicide (DCMU)-resistant mutant was used as donor DNA. DCMU resistance was also transferred to recipient cells by using restriction fragments of chromosomal DNA from DCMU-resistant mutants. This procedure allowed size classes of fragments to be assayed for the presence of the DCMU resistance gene.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chauvat F., Astier C., Vedel F., Joset-Espardellier F. Transformation in the cyanobacterium Synechococcus R2: improvement of efficiency; role of the pUH24 plasmid. Mol Gen Genet. 1983;191(1):39–45. doi: 10.1007/BF00330887. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Sherman L. A. A hybrid plasmid is a stable cloning vector for the cyanobacterium Anacystis nidulans R2. J Bacteriol. 1983 Sep;155(3):966–972. doi: 10.1128/jb.155.3.966-972.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Chlorophyll-protein organization of membranes from the cyanobacterium Anacystis nidulans. Arch Biochem Biophys. 1983 Jan;220(1):155–166. doi: 10.1016/0003-9861(83)90396-x. [DOI] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Influence of Iron Deprivation on the Membrane Composition of Anacystis nidulans. Plant Physiol. 1984 Jan;74(1):90–95. doi: 10.1104/pp.74.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Metronidazole and the isolation of temperature-sensitive photosynthetic mutants in cyanobacteria. J Bioenerg Biomembr. 1980 Aug;12(3-4):277–295. doi: 10.1007/BF00744689. [DOI] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Organization and Function of Chlorophyll in Membranes of Cyanobacteria during Iron Starvation. Plant Physiol. 1983 Oct;73(2):250–256. doi: 10.1104/pp.73.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Kuhlemeier C. J., Borrias W. E., van den Hondel C. A., van Arkel G. A. Vectors for cloning in cyanobacteria: construction and characterization of two recombinant plasmids capable of transformation of Escherichia coli K12 and Anacystis nidulans R2. Mol Gen Genet. 1981;184(2):249–254. doi: 10.1007/BF00272912. [DOI] [PubMed] [Google Scholar]
- Meagher R. B., Tait R. C., Betlach M., Boyer H. W. Protein expression in E. coli minicells by recombinant plasmids. Cell. 1977 Mar;10(3):521–536. doi: 10.1016/0092-8674(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Sherman L. A., van de Putte P. Construction of a hybrid plasmid capable of replication in the bacterium Escherichia coli and the cyanobacterium Anacystis nidulans. J Bacteriol. 1982 Apr;150(1):410–413. doi: 10.1128/jb.150.1.410-413.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Danner D. B., Deich R. A. Genetic transformation. Annu Rev Biochem. 1981;50:41–68. doi: 10.1146/annurev.bi.50.070181.000353. [DOI] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- van Nieuwenhoven M. H., Hellingwerf K. J., Venema G., Konings W. N. Role of proton motive force in genetic transformation of Bacillus subtilis. J Bacteriol. 1982 Aug;151(2):771–776. doi: 10.1128/jb.151.2.771-776.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hondel C. A., Keegstra W., Borrias W. E., van Arkel G. A. Homology of plasmids in strains of unicellular Cyanobacteria. Plasmid. 1979 Jul;2(3):323–333. doi: 10.1016/0147-619x(79)90016-7. [DOI] [PubMed] [Google Scholar]
- van den Hondel C. A., Verbeek S., van der Ende A., Weisbeek P. J., Borrias W. E., van Arkel G. A. Introduction of transposon Tn901 into a plasmid of Anacystis nidulans: preparation for cloning in cyanobacteria. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1570–1574. doi: 10.1073/pnas.77.3.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]



