Abstract
Mutants of Alcaligenes eutrophus H16 lacking catalytically active soluble hydrogenase (Hos-) grew very slowly lithoautotrophically with hydrogen. Mutants devoid of particulate hydrogenase activity (Hop-) were not affected in growth with hydrogen. The use of Hos- and Hop- mutants as donors of hydrogen-oxidizing ability in crosses with plasmid-free recipients impaired in both hydrogenases (Hox-) resulted in transconjugants which had inherited the plasmid and the phenotype of the donor. This indicates that the structural genes which code for the hydrogenases reside on plasmid pHG1. The Hox function of one class of Hox- mutants could not be restored by conjugation. These mutants exhibited a pleiotropic phenotype since they were unable to grow with hydrogen and also failed to grow heterotrophically with nitrate (Hox- Nit-). Nitrate was scarcely utilized as electron acceptor or as nitrogen source. Hox- Nit- mutants did not act as recipients but could act as donors of the Hox character. Transconjugants derived from those crosses were Hox+ Nit+, indicating that the mutation which leads to the Hox- Nit- phenotype maps on the chromosome. Apparently, the product of a chromosomal gene is involved in the expression of plasmid-encoded Hox genes. We observed that the elimination of plasmid pHG1 coincided with the occurrence of multiple resistances to various antibiotics. Since Hox+ transconjugate retained the antibiotic-resistant phenotype, we conclude that this property is not directly plasmid associated.
Full text
PDF

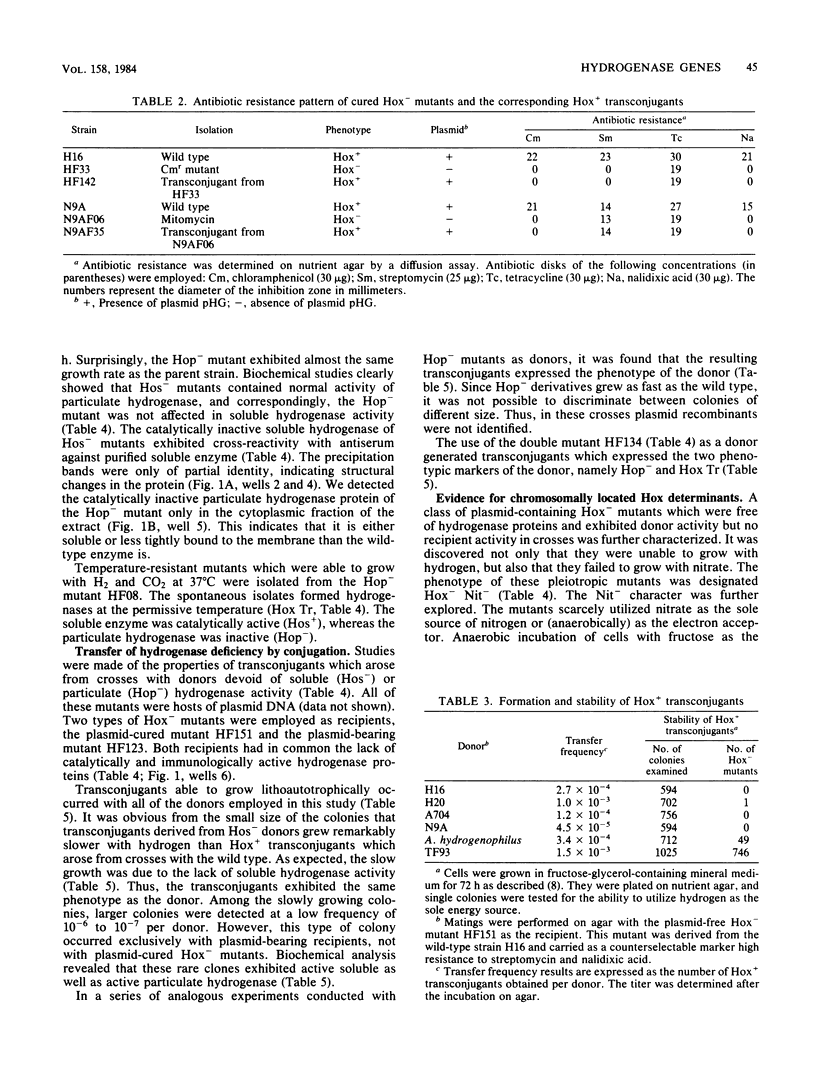

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowien B., Schlegel H. G. Physiology and biochemistry of aerobic hydrogen-oxidizing bacteria. Annu Rev Microbiol. 1981;35:405–452. doi: 10.1146/annurev.mi.35.100181.002201. [DOI] [PubMed] [Google Scholar]
- Cantrell M. A., Haugland R. A., Evans H. J. Construction of a Rhizobium japonicum gene bank and use in isolation of a hydrogen uptake gene. Proc Natl Acad Sci U S A. 1983 Jan;80(1):181–185. doi: 10.1073/pnas.80.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Heine E., Finck A., Friedrich C. G. Nickel requirement for active hydrogenase formation in Alcaligenes eutrophus. J Bacteriol. 1981 Mar;145(3):1144–1149. doi: 10.1128/jb.145.3.1144-1149.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Hogrefe C., Schlegel H. G. Naturally occurring genetic transfer of hydrogen-oxidizing ability between strains of Alcaligenes eutrophus. J Bacteriol. 1981 Jul;147(1):198–205. doi: 10.1128/jb.147.1.198-205.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich C. G. Depression of hydrogenase during limitation of electron donors and derepression of ribulosebisphosphate carboxylase during carbon limitation of Alcaligenes eutrophus. J Bacteriol. 1982 Jan;149(1):203–210. doi: 10.1128/jb.149.1.203-210.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich C. G., Friedrich B. Regulation of hydrogenase formation is temperature sensitive and plasmid coded in Alcaligenes eutrophus. J Bacteriol. 1983 Jan;153(1):176–181. doi: 10.1128/jb.153.1.176-181.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWE R. H., EVANS H. J. PREPARATION AND SOME PROPERTIES OF A SOLUBLE NITRATE REDUCTASE FROM RHIZOBIUM JAPONICUM. Biochim Biophys Acta. 1964 Jun 1;85:377–389. doi: 10.1016/0926-6569(64)90301-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pfitzner J. Ein Beitrag zum H2-O2-Oxidoreductasesystem von Hydrogenomonas eutropha Stamm H 16: Hydrogenasedefekte Mutanten. Zentralbl Bakteriol Orig A. 1974;228(1):121–127. [PubMed] [Google Scholar]
- SCHLEGEL H. G., KALTWASSER H., GOTTSCHALK G. [A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies]. Arch Mikrobiol. 1961;38:209–222. [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. Mutants of Alcaligenes eutrophus defective in autotrophic metabolism. Arch Microbiol. 1978 May 30;117(2):123–129. doi: 10.1007/BF00402299. [DOI] [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. The membrane-bound hydrogenase of Alcaligenes eutrophus. I. Solubilization, purification, and biochemical properties. Biochim Biophys Acta. 1979 Apr 12;567(2):315–324. doi: 10.1016/0005-2744(79)90117-7. [DOI] [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. The membrane-bound hydrogenase of Alcaligenes eutrophus: II. Localization and immunological comparison with other hydrogenase systems. Antonie Van Leeuwenhoek. 1980;46(1):1–14. doi: 10.1007/BF00422224. [DOI] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G. Purification and properties of soluble hydrogenase from Alcaligenes eutrophus H 16. Biochim Biophys Acta. 1976 Nov 8;452(1):66–80. doi: 10.1016/0005-2744(76)90058-9. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Urban M., Friedrich B. Mutagenesis of Alcaligenes eutrophus by insertion of the drug-resistance transposon Tn5. Arch Microbiol. 1982 May;131(3):203–207. doi: 10.1007/BF00405879. [DOI] [PubMed] [Google Scholar]