Abstract
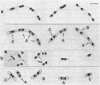
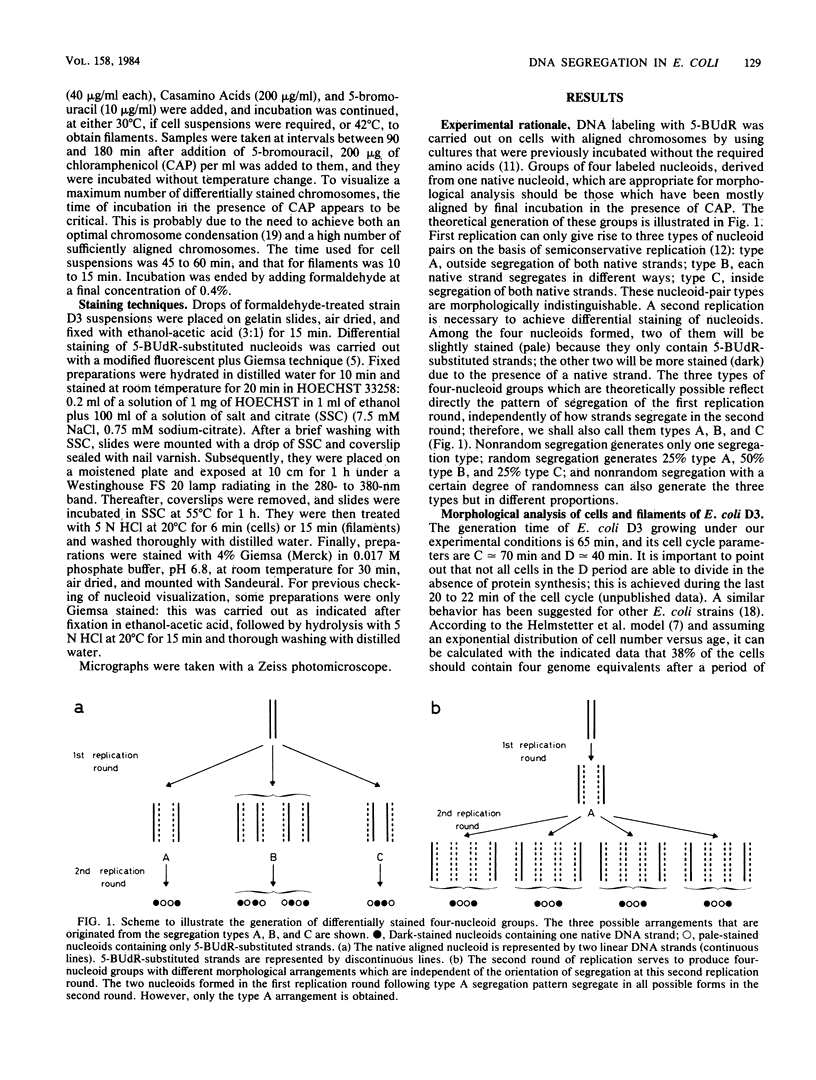
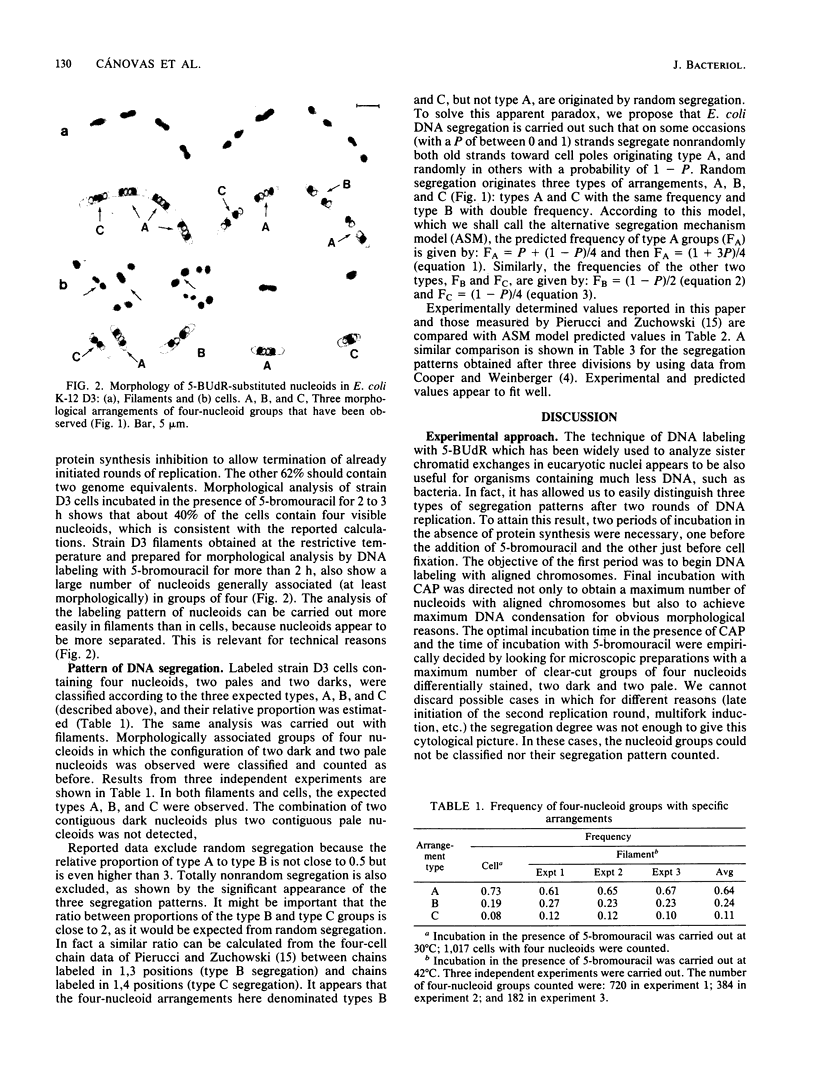
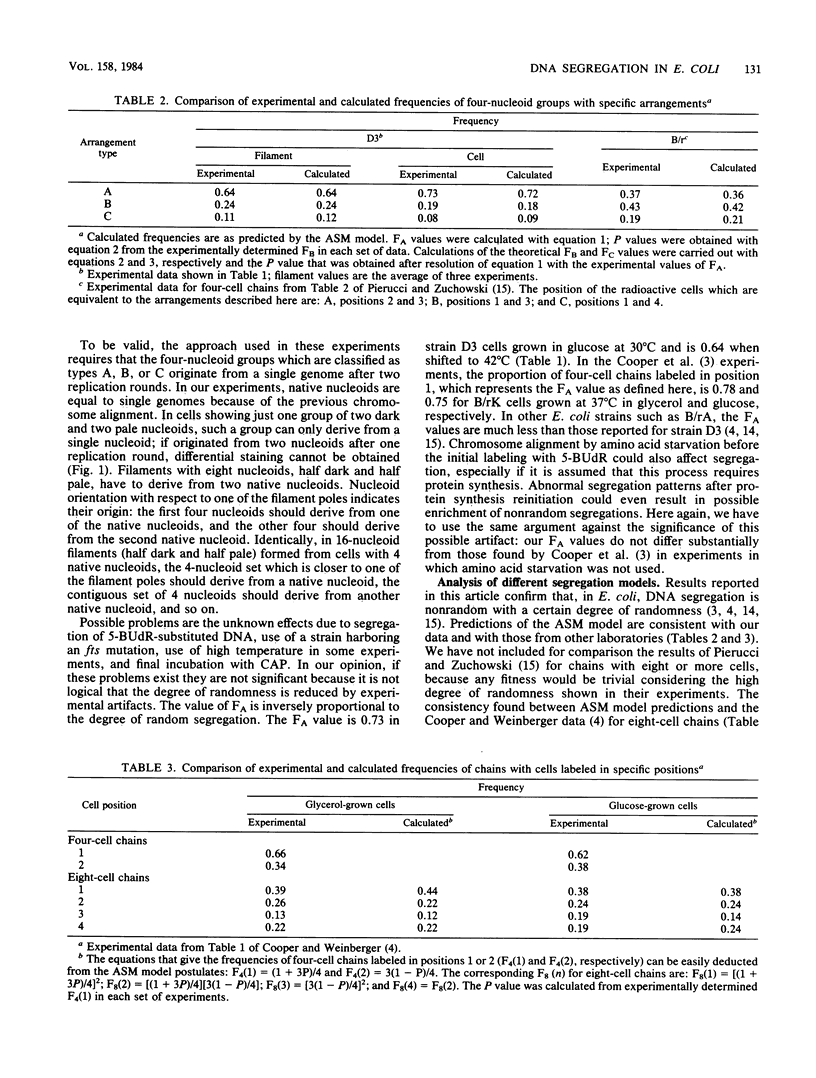
The pattern of segregation of DNA in Escherichia coli K-12 was analyzed by labeling replicating DNA with 5-bromodeoxyuridine followed by differential staining of nucleoids. Three types of visible arrangement were found in four-nucleoid groups derived from a native nucleoid after two replication rounds. Type A, segregation of both old strands toward cell poles, appeared with the highest frequency (0.6 to 0.8). Type B, segregation of one old strand toward the cell pole and the other toward the cell center, was twice as frequent as type C, segregation of both old strands toward the cell center. These results confirm previous data showing that DNA segregation in E. coli is nonrandom while presenting a certain degree of randomness. The proportions of the three indicated types of arrangement suggest a new probabilistic model to explain the observed segregation pattern. It is proposed that DNA strands segregate either nonrandomly, with a probability of between 0 and 1, or randomly. In nonrandom segregation, both old strands are always directed toward cell poles. Experimental data reported here or by other authors fit better with the predictions of this model than with those of other previously proposed proposed deterministic or probabilistic models.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chai N. C., Lark K. G. Cytological studies of deoxyribonucleic acid replication in Escherichia coli 15T-: replication at slow growth rates and after a shift-up into rich medium. J Bacteriol. 1970 Oct;104(1):401–409. doi: 10.1128/jb.104.1.401-409.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Schwimmer M., Scanlon S. Probabilistic behavior of DNA segregation in Escherichia coli. J Bacteriol. 1978 Apr;134(1):60–65. doi: 10.1128/jb.134.1.60-65.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Weinberger M. Medium-dependent variation of deoxyribonucleic acid segregation in Escherichia coli. J Bacteriol. 1977 Apr;130(1):118–127. doi: 10.1128/jb.130.1.118-127.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Gil G., Navarrete M. H. On the mechanism of differential Giemsa staining of BrdU-substituted chromatids. Chromosoma. 1982;86(3):375–382. doi: 10.1007/BF00292264. [DOI] [PubMed] [Google Scholar]
- Goyanes V. J., Schvartzman J. B. Insights on diplochromosome structure and behaviour. Chromosoma. 1981;83(1):93–102. doi: 10.1007/BF00286018. [DOI] [PubMed] [Google Scholar]
- Helmstetter C., Cooper S., Pierucci O., Revelas E. On the bacterial life sequence. Cold Spring Harb Symp Quant Biol. 1968;33:809–822. doi: 10.1101/sqb.1968.033.01.093. [DOI] [PubMed] [Google Scholar]
- Herreros B., Giannelli F. Spatial distribution of old and new chromatid sub-units and frequency of chromatid exchanges in induced human lymphocyte endoreduplications. Nature. 1967 Oct 21;216(5112):286–288. doi: 10.1038/216286a0. [DOI] [PubMed] [Google Scholar]
- Lin E. C., Hirota Y., Jacob F. On the process of cellular division in Escherichia coli. VI. Use of a methocel-autoradiographic method for the study of cellular division in Escherichia coli. J Bacteriol. 1971 Oct;108(1):375–385. doi: 10.1128/jb.108.1.375-385.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen H. A., Donachie W. D. Intracellular localization and effects on cell division of a plasmid blocked in deoxyribonucleic acid replication. J Bacteriol. 1977 Nov;132(2):392–397. doi: 10.1128/jb.132.2.392-397.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marunouchi T., Messer W. Replication of a specific terminal chromosome segment in Escherichia coli which is required for cell division. J Mol Biol. 1973 Jun 25;78(1):211–228. doi: 10.1016/0022-2836(73)90439-7. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W. THE REPLICATION OF DNA IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):671–682. doi: 10.1073/pnas.44.7.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci O., Helmstetter C. E. Chromosome segregation in Escherichia coli B/r at various growth rates. J Bacteriol. 1976 Dec;128(3):708–716. doi: 10.1128/jb.128.3.708-716.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci O., Zuchowski C. Non-random segregation of DNA strands in Escherichia coli B-r. J Mol Biol. 1973 Nov 5;80(3):477–503. doi: 10.1016/0022-2836(73)90417-8. [DOI] [PubMed] [Google Scholar]
- Ryter A., Hirota Y., Jacob F. DNA-membrane complex and nuclear segregation in bacteria. Cold Spring Harb Symp Quant Biol. 1968;33:669–676. doi: 10.1101/sqb.1968.033.01.076. [DOI] [PubMed] [Google Scholar]
- Tormo A., Martínez-Salas E., Vicente M. Involvement of the ftsA gene product in late stages of the Escherichia coli cell cycle. J Bacteriol. 1980 Feb;141(2):806–813. doi: 10.1128/jb.141.2.806-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldringh C. L., de Jong M. A., van den Berg W., Koppes L. Morphological analysis of the division cycle of two Escherichia coli substrains during slow growth. J Bacteriol. 1977 Jul;131(1):270–279. doi: 10.1128/jb.131.1.270-279.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman D. R., Carbonell A., Haga J. Y. Nucleoid condensation and cell division in Escherichia coli MX74T2 ts52 after inhibition of protein synthesis. J Bacteriol. 1973 Sep;115(3):1167–1178. doi: 10.1128/jb.115.3.1167-1178.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]