Abstract
"Phasmid" P4 is unusual in that it is capable of (i) temperate, (ii) lytic, helper-dependent, and (iii) plasmid modes of propagation. In this report we characterize most of the known P4 genetic functions as to their essential or nonessential roles in the stable maintenance of plasmid P4 vir1 (pP4 vir1 (pP4 vir1). We also identify growth conditions that can be used to stably maintain pP4 vir1 at any one of several different copy number levels (n = 1 to 3, n = 10 to 15, or n = 30 to 40). Analyses of a temperature-sensitive alpha derivative of pP4 vir1 show that shifting the temperature from 37 to 42 degrees C allows this mutant to maintain an integrated copy of the plasmid, whereas replication of free copies is repressed because of the nonpermissive condition for their DNA synthesis. Conversely, a shift from 42 to 37 degrees C can be used to reinstate plasmid propagation. The utility of the inducible states of pP4 vir1 is discussed with respect to its attributes as a vector with the potential for cloning inserts of DNA up to 33,000 base pairs in a wide range of bacterial hosts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI L. E. The effect of the inhibition of protein synthesis on the establishment of lysogeny. Virology. 1957 Aug;4(1):53–71. doi: 10.1016/0042-6822(57)90043-0. [DOI] [PubMed] [Google Scholar]
- Barrett K. J., Blinkova A., Arnold G. The bacteriophage P4 alpha gene is the structural gene for bacteriophage P4-induced RNA polymerase. J Virol. 1983 Oct;48(1):157–169. doi: 10.1128/jvi.48.1.157-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K. J., Gibbs W., Calendar R. A transcribing activity induced by satellite phage P4. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2986–2990. doi: 10.1073/pnas.69.10.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani G., Ljungquist E., Jagusztyn-Krynicka K., Jupp S. Defective particle assembly in wild type P2 bacteriophage and its correction by the lg mutation. J Gen Virol. 1978 Feb;38(2):251–261. doi: 10.1099/0022-1317-38-2-251. [DOI] [PubMed] [Google Scholar]
- Bertani L. E., Bertani G. Preparation and characterization of temperate, non-inducible bacteriophage P2 (host: Escherichia coli). J Gen Virol. 1970 Feb;6(2):201–212. doi: 10.1099/0022-1317-6-2-201. [DOI] [PubMed] [Google Scholar]
- Bowden D. W., Twersky R. S., Calendar R. Escherichia coli deoxyribonucleic acid synthesis mutants: their effect upon bacteriophage P2 and satellite bacteriophage P4 deoxyribonucleic acid synthesis. J Bacteriol. 1975 Oct;124(1):167–175. doi: 10.1128/jb.124.1.167-175.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calendar R., Ljungquist E., Deho G., Usher D. C., Goldstein R., Youderian P., Sironi G., Six E. W. Lysogenization by satellite phage P4. Virology. 1981 Aug;113(1):20–38. doi: 10.1016/0042-6822(81)90133-1. [DOI] [PubMed] [Google Scholar]
- Chandler M., Silver L., Frey J., Caro L. Suppression of an Escherichia coli dnaA mutation by the integrated R factor R100.1: generation of small plasmids after integration. J Bacteriol. 1977 Apr;130(1):303–311. doi: 10.1128/jb.130.1.303-311.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely S., Staudenbauer W. L. Regulation of plasmid DNA synthesis: isolation and characterization of copy number mutants of miniR6-5 and miniF plasmids. Mol Gen Genet. 1981;181(1):29–35. doi: 10.1007/BF00339001. [DOI] [PubMed] [Google Scholar]
- Geisselsoder J., Chidambaram M., Goldstein R. Transcriptional control of capsid size in the P2:P4 bacteriophage system. J Mol Biol. 1978 Dec 15;126(3):447–456. doi: 10.1016/0022-2836(78)90051-7. [DOI] [PubMed] [Google Scholar]
- Geisselsoder J., Sedivy J. M., Walsh R. B., Goldstein R. Capsid structure of satellite phage P4 and its P2 helper. J Ultrastruct Res. 1982 May;79(2):165–173. doi: 10.1016/s0022-5320(82)90028-4. [DOI] [PubMed] [Google Scholar]
- Geisselsoder J., Youdarian P., Dehò G., Chidambaram M., Goldstein R., Ljungquist E. Mutants of satellite virus P4 that cannot derepress their bacteriophage P2 helper. J Mol Biol. 1981 May 5;148(1):1–19. doi: 10.1016/0022-2836(81)90232-1. [DOI] [PubMed] [Google Scholar]
- Gibbs W., Goldstein R. N., Wiener R., Lindqvist B., Calendar R. Satellite bacteriophage P4: characterization of mutants in two essential genes. Virology. 1973 May;53(1):24–39. doi: 10.1016/0042-6822(73)90462-5. [DOI] [PubMed] [Google Scholar]
- Goldstein L., Thomas M., Davis R. W. EcoRI endonuclease cleavage map of bacteriophage P4-DNA. Virology. 1975 Aug;66(2):420–427. doi: 10.1016/0042-6822(75)90214-7. [DOI] [PubMed] [Google Scholar]
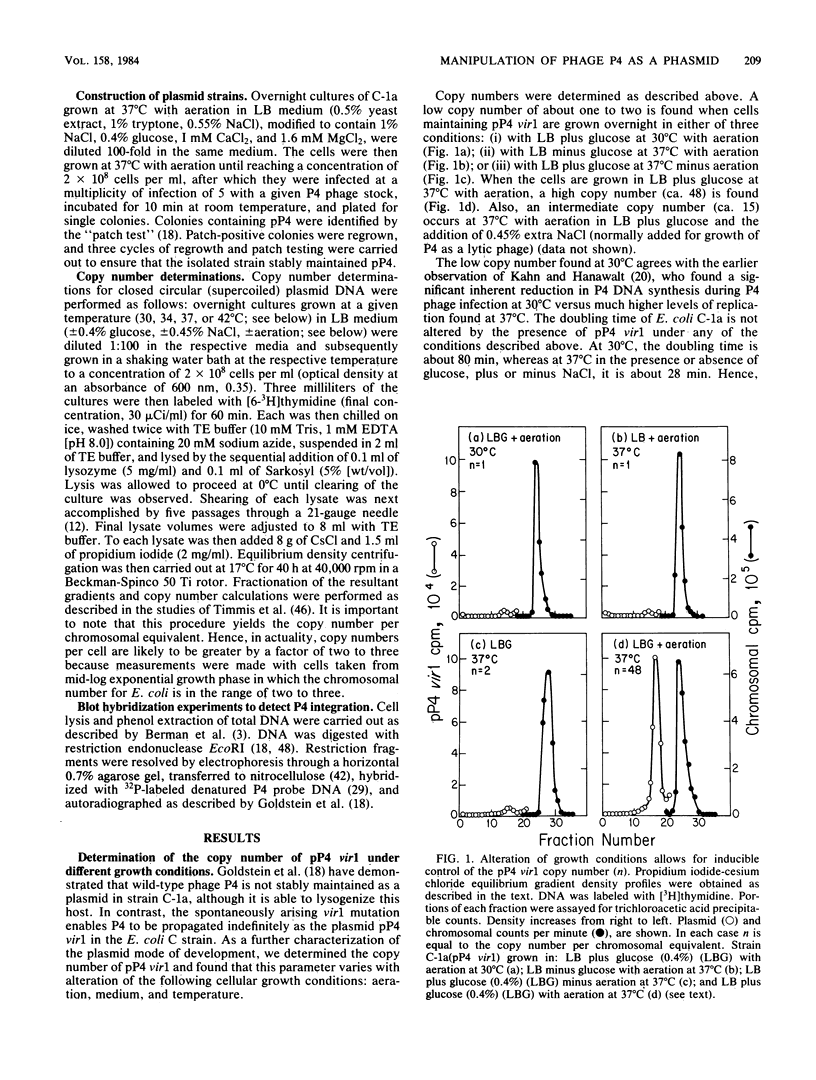
- Goldstein R., Sedivy J., Ljungquist E. Propagation of satellite phage P4 as a plasmid. Proc Natl Acad Sci U S A. 1982 Jan;79(2):515–519. doi: 10.1073/pnas.79.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman R. B., Schnös M., Simon L. D., Six E. W., Walker D. H., Jr Some morphological properties of P4 bacteriophage and P4 DNA. Virology. 1971 Apr;44(1):67–72. doi: 10.1016/0042-6822(71)90153-x. [DOI] [PubMed] [Google Scholar]
- Kahn M., Hanawalt P. Size distribution of DNA replicative intermediates in bacteriophage P4 and in Escherichia coli. J Mol Biol. 1979 Mar 15;128(4):501–525. doi: 10.1016/0022-2836(79)90290-0. [DOI] [PubMed] [Google Scholar]
- Kahn M., Helinski D. R. Construction of a novel plasmid-phage hybrid: use of the hybrid to demonstrate ColE1 DNA replication in vivo in the absence of a ColE1-specified protein. Proc Natl Acad Sci U S A. 1978 May;75(5):2200–2204. doi: 10.1073/pnas.75.5.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M., Hopkins A. Restriction endonuclease cleavage map of bacteriophage P4 DNA. Virology. 1978 Apr;85(2):359–363. doi: 10.1016/0042-6822(78)90444-0. [DOI] [PubMed] [Google Scholar]
- Kahn M., Ow D., Sauer B., Rabinowitz A., Calendar R. Genetic analysis of bacteriophage P4 using P4-plasmid ColE1 hybrids. Mol Gen Genet. 1980 Feb;177(3):399–412. doi: 10.1007/BF00271478. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Yanofsky C. Polarity suppressors defective in transcription termination at the attenuator of the tryptophan operon of Escherichia coli have altered rho factor. J Mol Biol. 1976 Sep 15;106(2):231–241. doi: 10.1016/0022-2836(76)90082-6. [DOI] [PubMed] [Google Scholar]
- Lindahl G. On the control of transcription in bacteriophage P2. Virology. 1971 Dec;46(3):620–633. doi: 10.1016/0042-6822(71)90065-1. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H. Expression of phage transcription in P2 lysogens infected with helper-dependent coliphage P4. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2752–2755. doi: 10.1073/pnas.71.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist B. H., Six E. W. Replication of bacteriophage P4 DNA in a nonlysogenic host. Virology. 1971 Jan;43(1):1–7. doi: 10.1016/0042-6822(71)90218-2. [DOI] [PubMed] [Google Scholar]
- Louarn J. M. Size distribution and molecular polarity of nascent DNA in a temperature-sensitive dna G mutant of Escherichia coli. Mol Gen Genet. 1974;133(3):193–200. doi: 10.1007/BF00267668. [DOI] [PubMed] [Google Scholar]
- Matsubara K., Kaiser A. D. Lambda dv: an autonomously replicating DNA fragment. Cold Spring Harb Symp Quant Biol. 1968;33:769–775. doi: 10.1101/sqb.1968.033.01.088. [DOI] [PubMed] [Google Scholar]
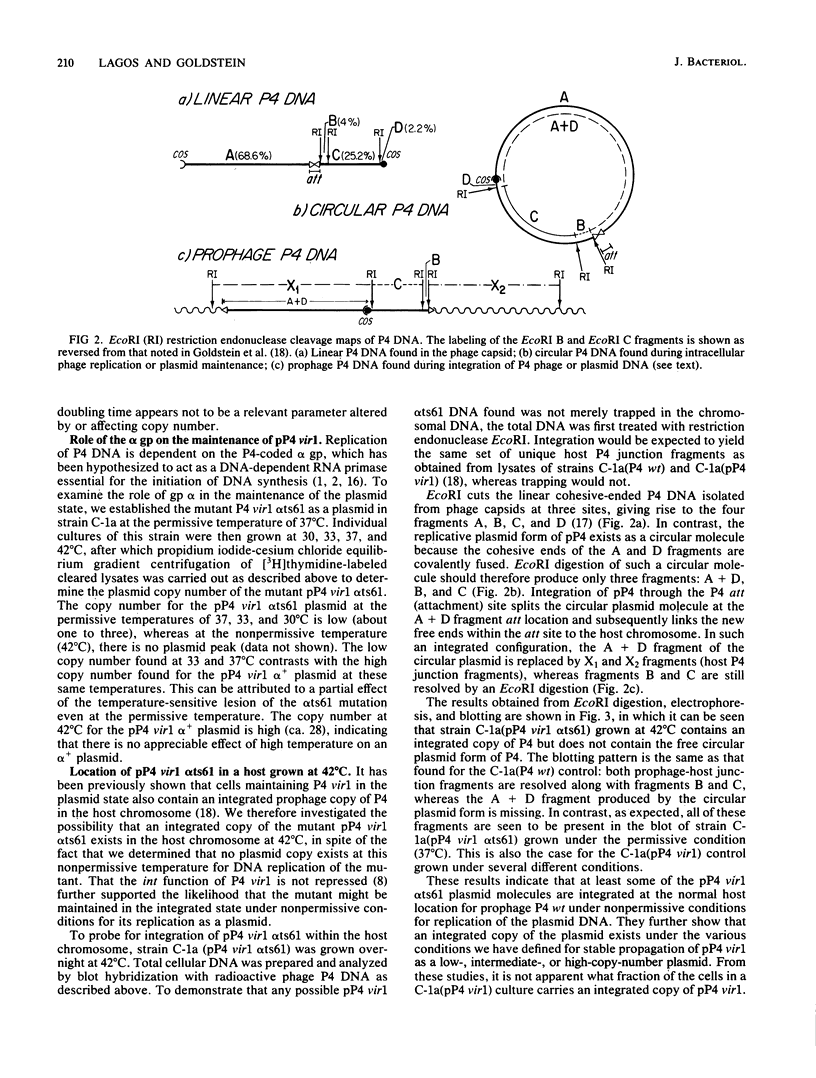
- Ow D. W., Ausubel F. M. Recombinant P4 bacteriophages propagate as viable lytic phages or as autonomous plasmids in Klebsiella pneumoniae. Mol Gen Genet. 1980;180(1):165–175. doi: 10.1007/BF00267366. [DOI] [PubMed] [Google Scholar]
- Pruss G. J., Wang J. C., Calendar R. In vitro packaging of covalently closed circular monomers of bacteriophage DNA. J Mol Biol. 1975 Nov 5;98(3):465–478. doi: 10.1016/s0022-2836(75)80080-5. [DOI] [PubMed] [Google Scholar]
- Pruss G., Goldstein R. N., Calendar R. In vitro packaging of satellite phage P4 DNA. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2367–2371. doi: 10.1073/pnas.71.6.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki I., Bertani G. Growth abnormalities in Hfr derivatives of Escherichia coli strain C. J Gen Microbiol. 1965 Sep;40(3):365–376. doi: 10.1099/00221287-40-3-365. [DOI] [PubMed] [Google Scholar]
- Sauer B., Ow D., Ling L., Calendar R. Mutants of satellite bacteriophage P4 that are defective in the suppression of transcriptional polarity. J Mol Biol. 1981 Jan 5;145(1):29–46. doi: 10.1016/0022-2836(81)90333-8. [DOI] [PubMed] [Google Scholar]
- Scott J. R. Immunity and repression in bacteriophages P1 and P7. Curr Top Microbiol Immunol. 1980;90:49–65. doi: 10.1007/978-3-642-67717-5_3. [DOI] [PubMed] [Google Scholar]
- Shore D., Dehò G., Tsipis J., Goldstein R. Determination of capsid size by satellite bacteriophage P4. Proc Natl Acad Sci U S A. 1978 Jan;75(1):400–404. doi: 10.1073/pnas.75.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi G. Mutants of Escherichia coli unable to be lysogenized by the temperate bacteriophage P2. Virology. 1969 Feb;37(2):163–176. doi: 10.1016/0042-6822(69)90196-2. [DOI] [PubMed] [Google Scholar]
- Six E. W., Klug C. A. Bacteriophage P4: a satellite virus depending on a helper such as prophage P2. Virology. 1973 Feb;51(2):327–344. doi: 10.1016/0042-6822(73)90432-7. [DOI] [PubMed] [Google Scholar]
- Six E. W., Lindqvist B. H. Multiplication of bacteriophage P4 in the absence of replication of the DNA of its helper. Virology. 1971 Jan;43(1):8–15. doi: 10.1016/0042-6822(71)90219-4. [DOI] [PubMed] [Google Scholar]
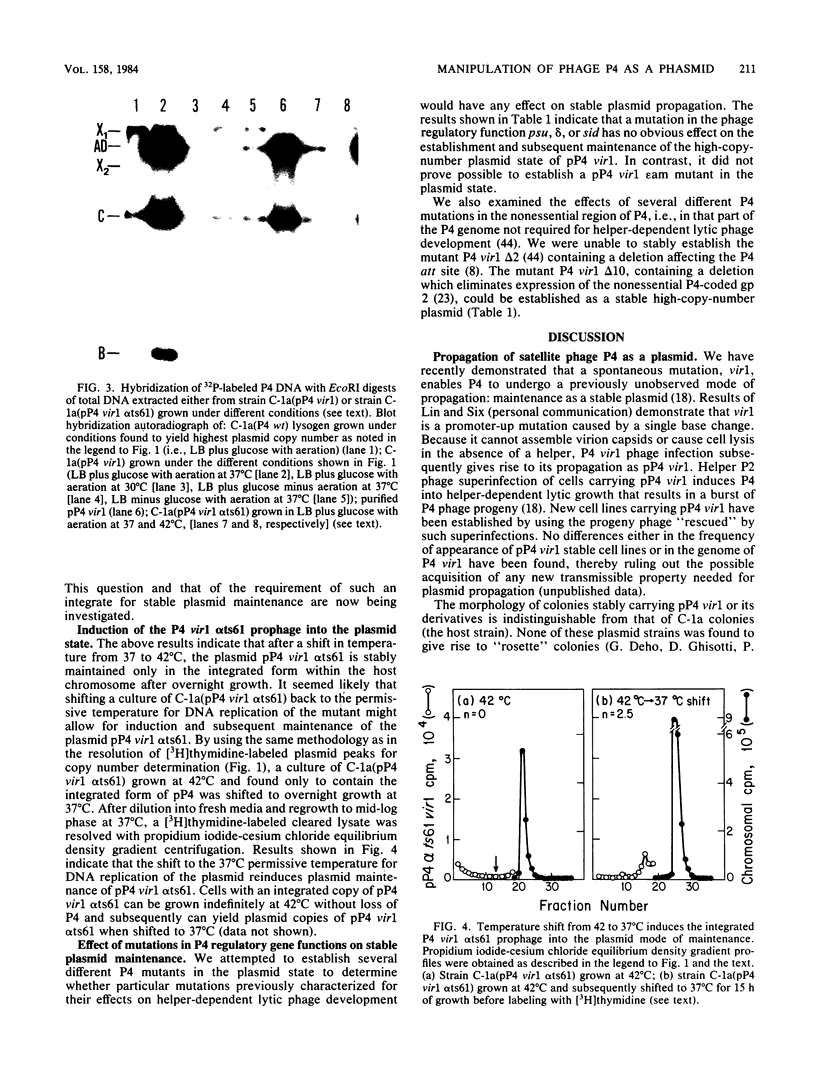
- Six E. W. The helper dependence of satellite bacteriophage P4: which gene functions of bacteriophage P2 are needed by P4? Virology. 1975 Sep;67(1):249–263. doi: 10.1016/0042-6822(75)90422-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Souza L., Calendar R., Six E. W., Lindqvist B. H. A transactivation mutant of satellite phage P4. Virology. 1977 Aug;81(1):81–90. doi: 10.1016/0042-6822(77)90060-5. [DOI] [PubMed] [Google Scholar]
- Souza L., Geisselsoder J., Hopkins A., Calender R. Physical mapping of the satellite phage P4 genome. Virology. 1978 Apr;85(2):335–342. doi: 10.1016/0042-6822(78)90442-7. [DOI] [PubMed] [Google Scholar]
- Sunshine M. G., Thorn M., Gibbs W., Calendar R., Kelly B. P2 phage amber mutants: characterization by use of a polarity suppressor. Virology. 1971 Dec;46(3):691–702. doi: 10.1016/0042-6822(71)90071-7. [DOI] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Utilization of two distinct modes of replication by a hybrid plasmid constructed in vitro from separate replicons. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4556–4560. doi: 10.1073/pnas.71.11.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Westö A., Ljungquist E. A restriction endonuclease cleavage map of bacteriophage P2. Mol Gen Genet. 1979 Mar 9;171(1):91–102. doi: 10.1007/BF00274019. [DOI] [PubMed] [Google Scholar]
- Zyskind J. W., Deen L. T., Smith D. W. Temporal sequence of events during the initiation process in Escherichia coli deoxyribonucleic acid replication: roles of the dnaA and dnaC gene products and ribonucleic acid polymerase. J Bacteriol. 1977 Mar;129(3):1466–1475. doi: 10.1128/jb.129.3.1466-1475.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]