Abstract
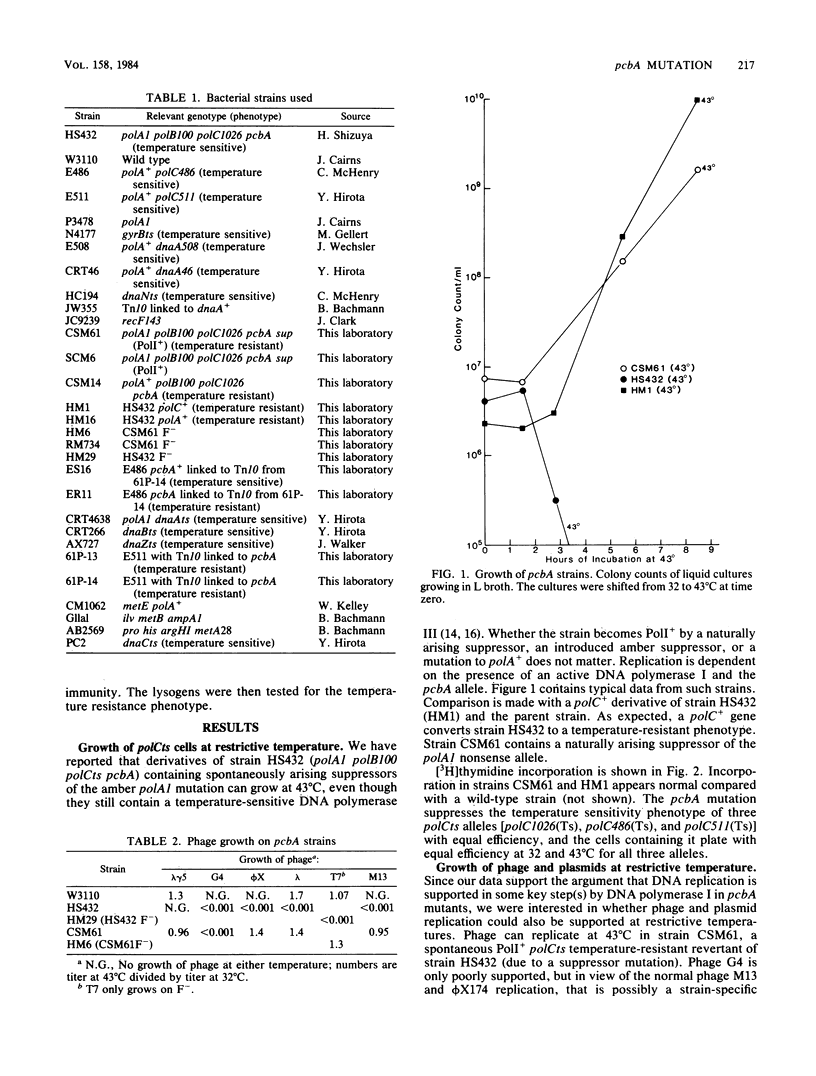
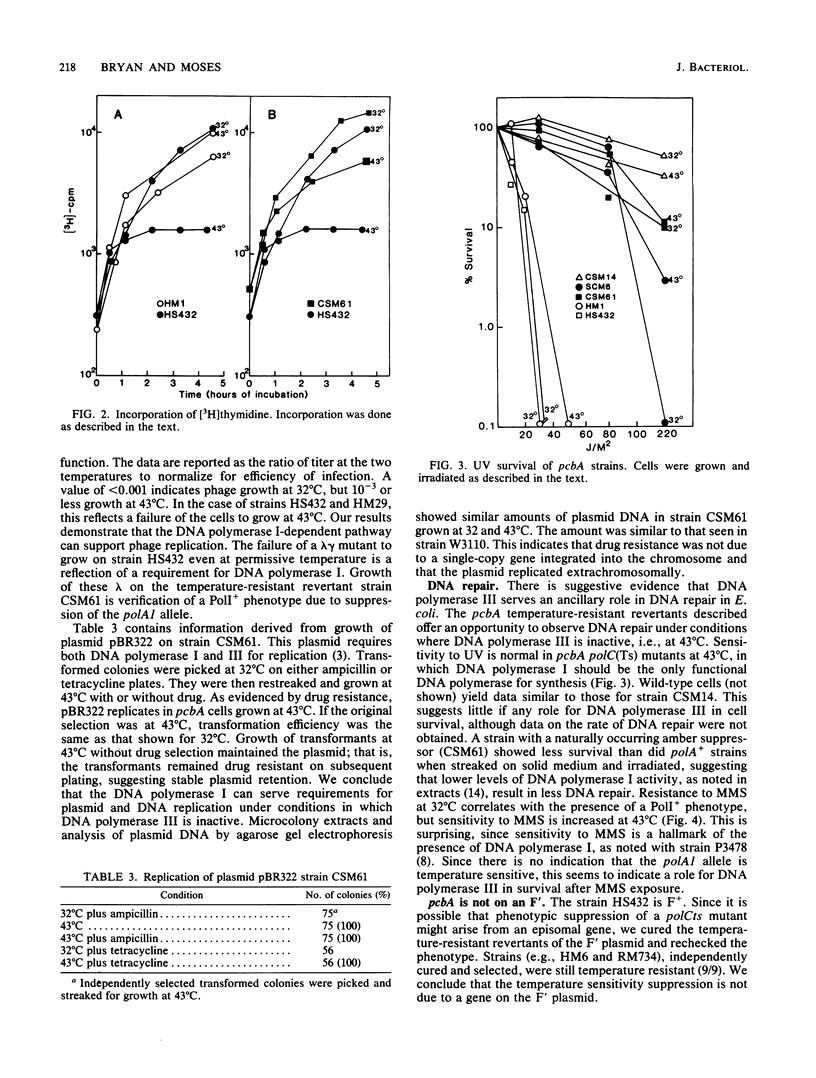
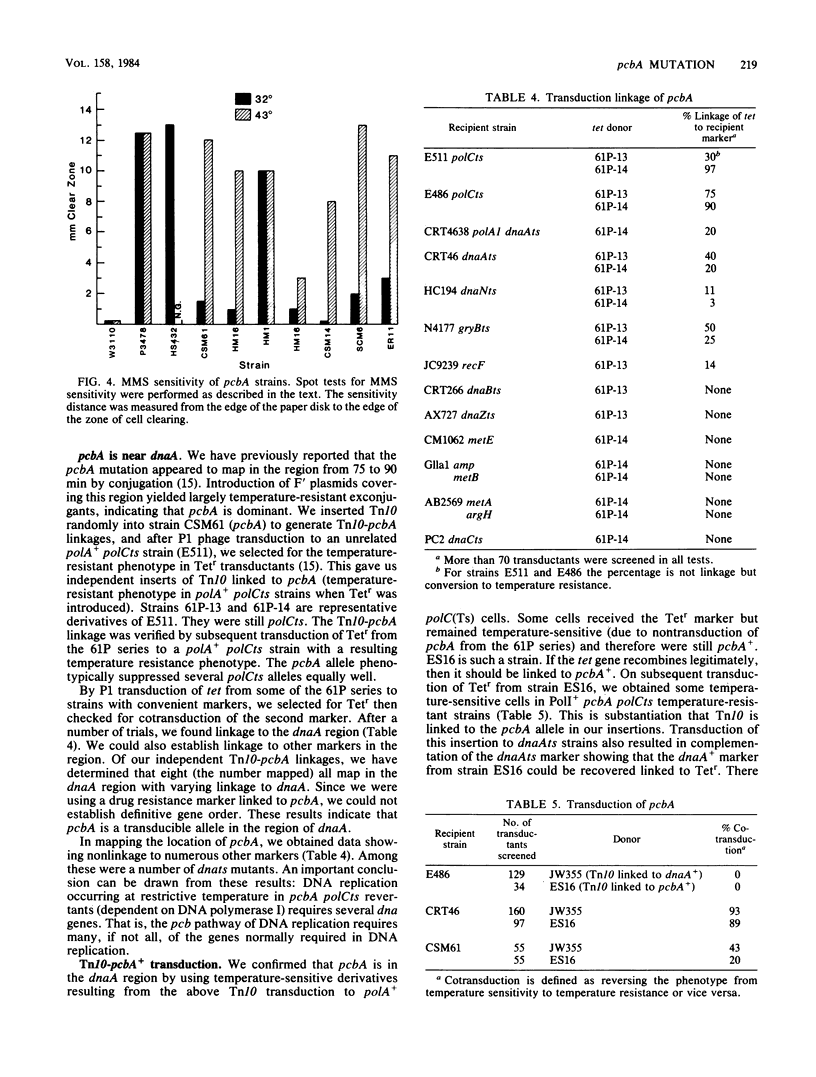
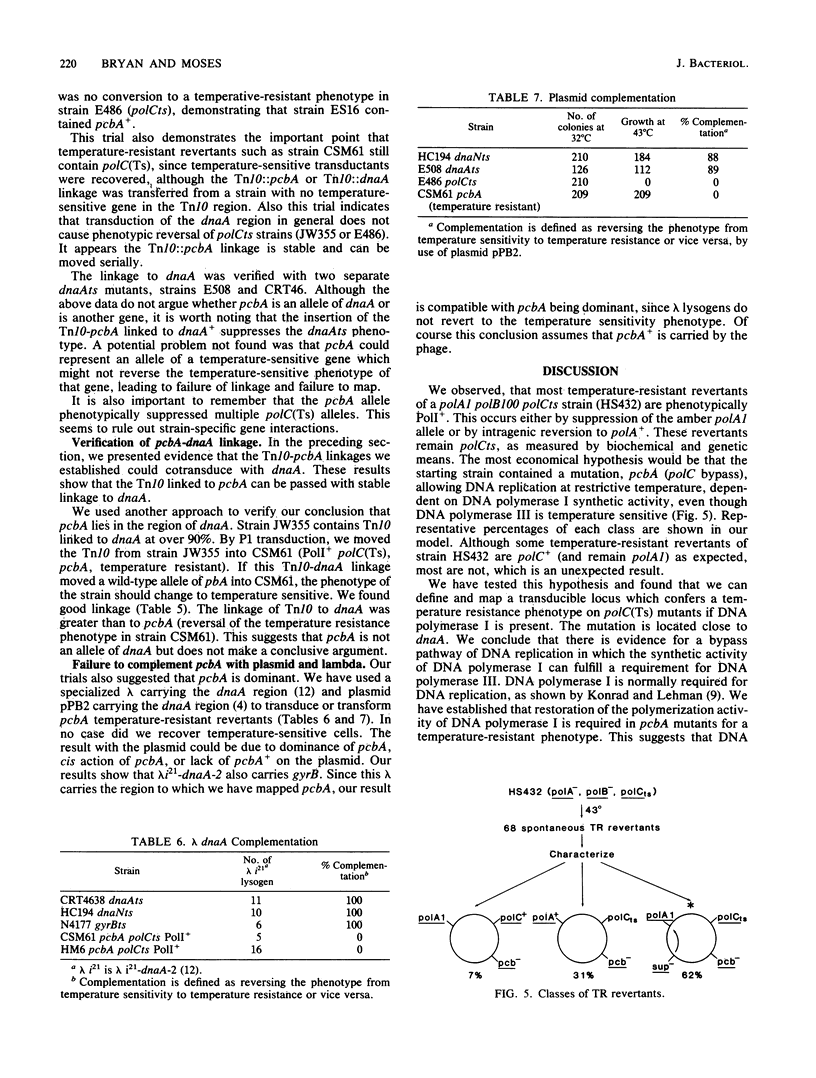
Many temperature-resistant revertants of a polA1 polB polCts (HS432) strain are PolI+ (by either suppression of the polA1 amber allele or intragenic reversion) but remain polCts (contain a temperature-sensitive DNA polymerase III). It appears that DNA replication in such temperature-resistant revertants depends on an extragenic mutation, pcbA, already present in the parent strain and not linked to any of the DNA polymerase loci. This allele allows DNA replication dependent on DNA polymerase I and bypasses a temperature-sensitive DNA polymerase III (polC bypass), so that reversion to PolI+ makes the strain temperature resistant. This pathway of DNA replication also supports phage and plasmid DNA replication. At restrictive temperature, these mutants display a normal response to UV irradiation but show increased sensitivity to the alkylating agent methyl methanesulfonate. We have located pcbA linked to dnaA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutin L., Achtman M. Two Escherichia coli chromosomal cistrons, sfrA and sfrB, which are needed for expression of F factor tra functions. J Bacteriol. 1979 Sep;139(3):730–737. doi: 10.1128/jb.139.3.730-737.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinkowa A., Walker J. R. Interactions of DNA replication factors in vivo as detected by introduction of suppressor alleles of dnaA into other temperature-sensitive dna mutants. J Bacteriol. 1983 Jan;153(1):535–538. doi: 10.1128/jb.153.1.535-538.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Burgers P. M., Kornberg A., Sakakibara Y. The dnaN gene codes for the beta subunit of DNA polymerase III holoenzyme of escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5391–5395. doi: 10.1073/pnas.78.9.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Fay P. J., Johanson K. O., McHenry C. S., Bambara R. A. Size classes of products synthesized processively by DNA polymerase III and DNA polymerase III holoenzyme of Escherichia coli. J Biol Chem. 1981 Jan 25;256(2):976–983. [PubMed] [Google Scholar]
- Gefter M. L., Hirota Y., Kornberg T., Wechsler J. A., Barnoux C. Analysis of DNA polymerases II and 3 in mutants of Escherichia coli thermosensitive for DNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3150–3153. doi: 10.1073/pnas.68.12.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- Konrad E. B., Lehman I. R. A conditional lethal mutant of Escherichia coli K12 defective in the 5' leads to 3' exonuclease associated with DNA polymerase I. Proc Natl Acad Sci U S A. 1974 May;71(5):2048–2051. doi: 10.1073/pnas.71.5.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara N., Uchida H. Functional cooperation of the dnaE and dnaN gene products in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5764–5767. doi: 10.1073/pnas.78.9.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Kimura M., Hiraga S., Nagata T., Yura T. Cloning and physical mapping of the dnaA region of the Escherichia coli chromosome. J Bacteriol. 1979 Dec;140(3):817–824. doi: 10.1128/jb.140.3.817-824.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation in Escherichia coli: cryogenic preservation of competent cells. J Bacteriol. 1977 Oct;132(1):349–351. doi: 10.1128/jb.132.1.349-351.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa O., Bryan S. K., Moses R. E. Alternate pathways of DNA replication: DNA polymerase I-dependent replication. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7024–7027. doi: 10.1073/pnas.78.11.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa O., Bryan S. K., Moses R. E. Replication at restrictive temperatures in Escherichia coli containing a polCts mutation. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5572–5576. doi: 10.1073/pnas.76.11.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G. Prokaryotic DNA replication systems. Annu Rev Biochem. 1983;52:581–615. doi: 10.1146/annurev.bi.52.070183.003053. [DOI] [PubMed] [Google Scholar]



