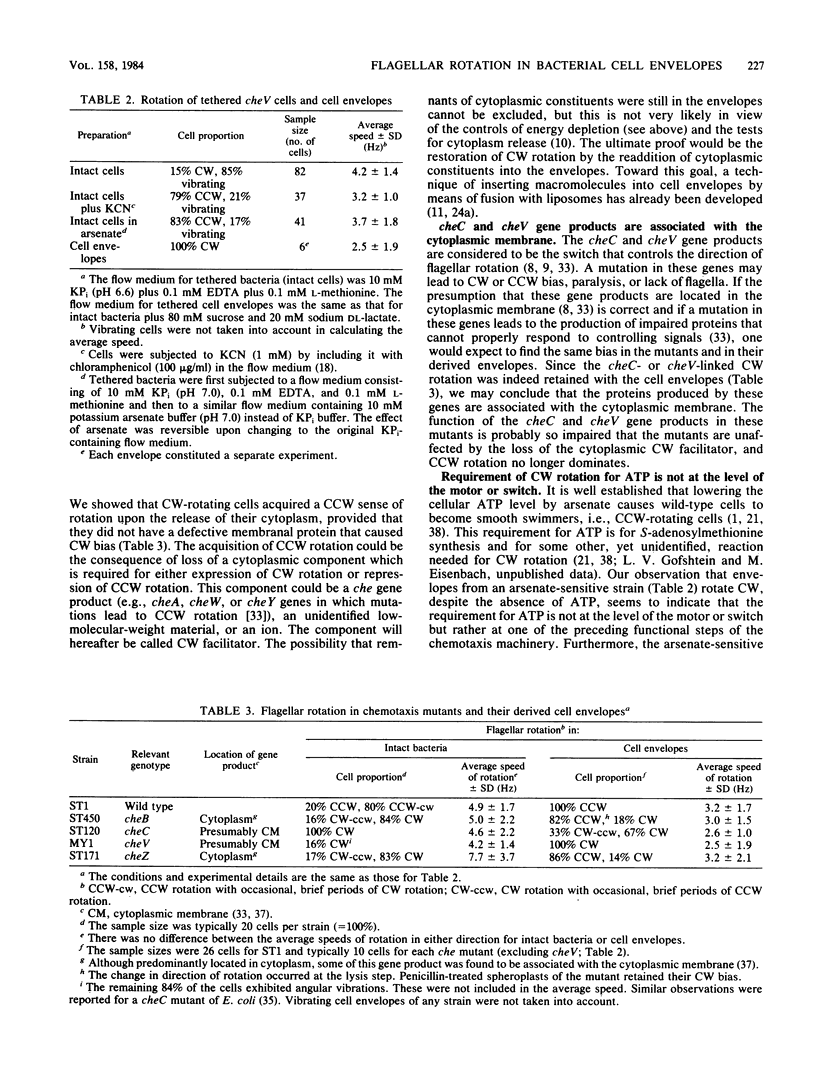
Abstract
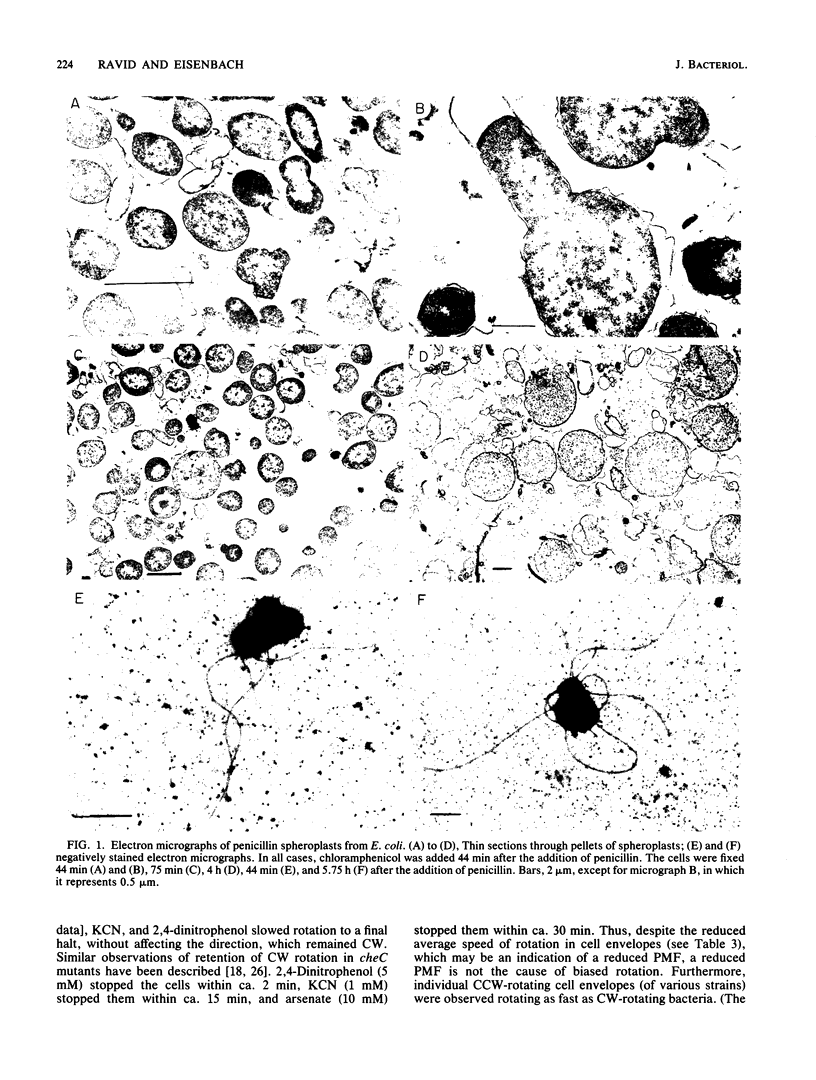
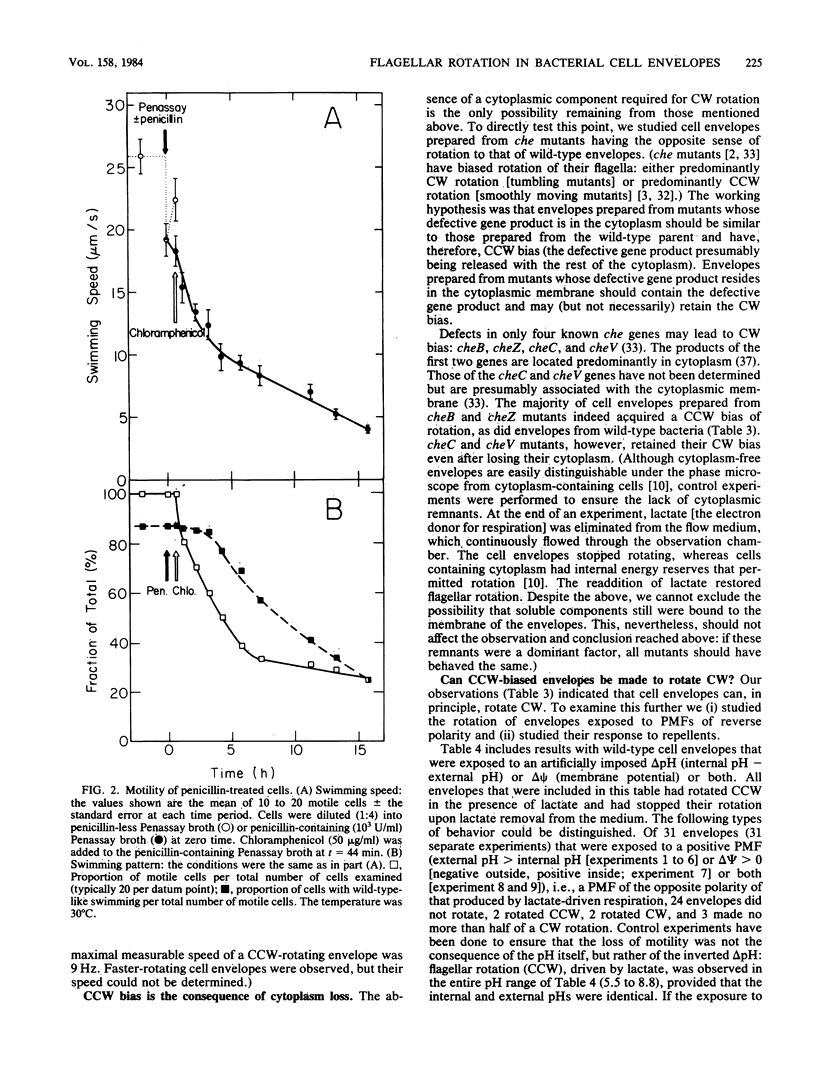
Cell envelopes with functional flagella, isolated from wild-type strains of Escherichia coli and Salmonella typhimurium by formation of spheroplasts with penicillin and subsequent osmotic lysis, demonstrate counterclockwise (CCW)-biased rotation when energized with an electron donor for respiration, DL-lactate. Since the direction of flagellar rotation in bacteria is central to the expression of chemotaxis, we studied the cause of this bias. Our main observations were: (i) spheroplasts acquired a clockwise (CW) bias if instead of being lysed they were further incubated with penicillin; (ii) repellents temporarily caused CW rotation of tethered bacteria and spheroplasts but not of their derived cell envelopes; (iii) deenergizing CW-rotating cheV bacteria by KCN or arsenate treatment caused CCW bias; (iv) cell envelopes isolated from CW-rotating cheC and cheV mutants retained the CW bias, unlike envelopes isolated from cheB and cheZ mutants, which upon cytoplasmic release lost this bias and acquired CCW bias; and (v) an inwardly directed, artificially induced proton current rotated tethered envelopes in CCW direction, but an outwardly directed current was unable to rotate the envelopes. It is concluded that (i) a cytoplasmic constituent is required for the expression of CW rotation (or repression of CCW rotation) in strains which are not defective in the switch; (ii) in the absence of this cytoplasmic constituent, the motor is not reversible in such strains, and it probably is mechanically constricted so as to permit CCW sense of rotation only; (iii) the requirement of CW rotation for ATP is not at the level of the motor or the switch but at one of the preceding functional steps of the chemotaxis machinery; (iv) the cheC and cheV gene products are associated with the cytoplasmic membrane; and (v) direct interaction between the switch-motor system and the repellent sensors is improbable.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai T. Effect of arsenate on chemotactic behavior of Escherichia coli. J Bacteriol. 1981 Feb;145(2):803–807. doi: 10.1128/jb.145.2.803-807.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J., Dahl M. M. Nonchemotactic mutants of Escherichia coli. J Bacteriol. 1967 Jan;93(1):390–398. doi: 10.1128/jb.93.1.390-398.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswad D., Koshland D. E., Jr Isolation, characterization and complementation of Salmonella typhimurium chemotaxis mutants. J Mol Biol. 1975 Sep 15;97(2):225–235. doi: 10.1016/s0022-2836(75)80036-2. [DOI] [PubMed] [Google Scholar]
- Bar Tana J., Howlett B. J., Koshland D. E., Jr Flagellar formation in Escherichia coli electron transport mutants. J Bacteriol. 1977 May;130(2):787–792. doi: 10.1128/jb.130.2.787-792.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Anderson R. A. Bacteria swim by rotating their flagellar filaments. Nature. 1973 Oct 19;245(5425):380–382. doi: 10.1038/245380a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Manson M. D., Conley M. P. Dynamics and energetics of flagellar rotation in bacteria. Symp Soc Exp Biol. 1982;35:1–31. [PubMed] [Google Scholar]
- DeFranco A. L., Koshland D. E., Jr Construction and behavior of strains with mutations in two chemotaxis genes. J Bacteriol. 1982 Jun;150(3):1297–1301. doi: 10.1128/jb.150.3.1297-1301.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean G. E., Aizawa S. I., Macnab R. M. flaAII (motC, cheV) of Salmonella typhimurium is a structural gene involved in energization and switching of the flagellar motor. J Bacteriol. 1983 Apr;154(1):84–91. doi: 10.1128/jb.154.1.84-91.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbach M., Adler J. Bacterial cell envelopes with functional flagella. J Biol Chem. 1981 Aug 25;256(16):8807–8814. [PubMed] [Google Scholar]
- Engström P., Hazelbauer G. L. Methyl-accepting chemotaxis proteins are distributed in the membrane independently from basal ends of bacterial flagella. Biochim Biophys Acta. 1982 Mar 23;686(1):19–26. doi: 10.1016/0005-2736(82)90147-x. [DOI] [PubMed] [Google Scholar]
- Goy M. F., Springer M. S., Adler J. Failure of sensory adaptation in bacterial mutants that are defective in a protein methylation reaction. Cell. 1978 Dec;15(4):1231–1240. doi: 10.1016/0092-8674(78)90049-1. [DOI] [PubMed] [Google Scholar]
- Khan S., Berg H. C. Isotope and thermal effects in chemiosmotic coupling to the flagellar motor of Streptococcus. Cell. 1983 Mar;32(3):913–919. doi: 10.1016/0092-8674(83)90076-4. [DOI] [PubMed] [Google Scholar]
- Khan S., Macnab R. M. Proton chemical potential, proton electrical potential and bacterial motility. J Mol Biol. 1980 Apr 15;138(3):599–614. doi: 10.1016/s0022-2836(80)80019-2. [DOI] [PubMed] [Google Scholar]
- Khan S., Macnab R. M. The steady-state counterclockwise/clockwise ratio of bacterial flagellar motors is regulated by protonmotive force. J Mol Biol. 1980 Apr 15;138(3):563–597. doi: 10.1016/s0022-2836(80)80018-0. [DOI] [PubMed] [Google Scholar]
- Kihara M., Macnab R. M. Cytoplasmic pH mediates pH taxis and weak-acid repellent taxis of bacteria. J Bacteriol. 1981 Mar;145(3):1209–1221. doi: 10.1128/jb.145.3.1209-1221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh H. Tumbling chemotaxis mutants of Escherichia coli: possible gene-dependent effect of methionine starvation. J Bacteriol. 1980 May;142(2):527–534. doi: 10.1128/jb.142.2.527-534.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S. H., Adler J., Gargus J. J., Hogg R. W. Chemomechanical coupling without ATP: the source of energy for motility and chemotaxis in bacteria. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1239–1243. doi: 10.1073/pnas.71.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S. H., Reader R. W., Kort E. N., Tso W. W., Adler J. Change in direction of flagellar rotation is the basis of the chemotactic response in Escherichia coli. Nature. 1974 May 3;249(452):74–77. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- Lederberg J. BACTERIAL PROTOPLASTS INDUCED BY PENICILLIN. Proc Natl Acad Sci U S A. 1956 Sep;42(9):574–577. doi: 10.1073/pnas.42.9.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelkes P. I., Klein L., Marikovsky Y., Eisenbach M. Liposome-mediated transfer of macromolecules into flagellated cell envelopes from bacteria. Biochemistry. 1984 Jan 31;23(3):563–568. doi: 10.1021/bi00298a026. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Han D. P. Asynchronous switching of flagellar motors on a single bacterial cell. Cell. 1983 Jan;32(1):109–117. doi: 10.1016/0092-8674(83)90501-9. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Koshland D. E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Ornston M. K. Normal-to-curly flagellar transitions and their role in bacterial tumbling. Stabilization of an alternative quaternary structure by mechanical force. J Mol Biol. 1977 May 5;112(1):1–30. doi: 10.1016/s0022-2836(77)80153-8. [DOI] [PubMed] [Google Scholar]
- Macnab R. M. Sensory reception in bacteria. Symp Soc Exp Biol. 1982;35:77–104. [PubMed] [Google Scholar]
- Manson M. D., Tedesco P. M., Berg H. C. Energetics of flagellar rotation in bacteria. J Mol Biol. 1980 Apr 15;138(3):541–561. doi: 10.1016/s0022-2836(80)80017-9. [DOI] [PubMed] [Google Scholar]
- Manson M. D., Tedesco P., Berg H. C., Harold F. M., Van der Drift C. A protonmotive force drives bacterial flagella. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3060–3064. doi: 10.1073/pnas.74.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsura S., Shioi J., Imae Y. Motility in Bacillus subtilis driven by an artificial protonmotive force. FEBS Lett. 1977 Oct 15;82(2):187–190. doi: 10.1016/0014-5793(77)80581-4. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S. Data processing by the chemotaxis machinery of Escherichia coli. Nature. 1974 Nov 22;252(5481):317–319. doi: 10.1038/252317a0. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S., Parker S. R., Talbert P. B., Houts S. E. Interactions between chemotaxis genes and flagellar genes in Escherichia coli. J Bacteriol. 1983 Jul;155(1):265–274. doi: 10.1128/jb.155.1.265-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravid S., Eisenbach M. Correlation between bacteriophage chi adsorption and mode of flagellar rotation of Escherichia coli chemotaxis mutants. J Bacteriol. 1983 May;154(2):604–611. doi: 10.1128/jb.154.2.604-611.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske D. R., Adler J. Change in intracellular pH of Escherichia coli mediates the chemotactic response to certain attractants and repellents. J Bacteriol. 1981 Mar;145(3):1196–1208. doi: 10.1128/jb.145.3.1196-1208.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway H. G., Silverman M., Simon M. I. Localization of proteins controlling motility and chemotaxis in Escherichia coli. J Bacteriol. 1977 Nov;132(2):657–665. doi: 10.1128/jb.132.2.657-665.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi J. I., Galloway R. J., Niwano M., Chinnock R. E., Taylor B. L. Requirement of ATP in bacterial chemotaxis. J Biol Chem. 1982 Jul 25;257(14):7969–7975. [PubMed] [Google Scholar]
- Silverman M., Simon M. Flagellar rotation and the mechanism of bacterial motility. Nature. 1974 May 3;249(452):73–74. doi: 10.1038/249073a0. [DOI] [PubMed] [Google Scholar]
- Springer M. S., Kort E. N., Larsen S. H., Ordal G. W., Reader R. W., Adler J. Role of methionine in bacterial chemotaxis: requirement for tumbling and involvement in information processing. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4640–4644. doi: 10.1073/pnas.72.11.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thipayathasana P., Valentine R. The requirement for energy transducing ATPase for anaerobic motility in Escherichia coli. Biochim Biophys Acta. 1974 Jun 28;347(3):464–468. doi: 10.1016/0005-2728(74)90083-8. [DOI] [PubMed] [Google Scholar]
- Tsang N., Macnab R., Koshland D. E., Jr Common mechanism for repellents and attractants in bacterial chemotaxis. Science. 1973 Jul 6;181(4094):60–63. doi: 10.1126/science.181.4094.60. [DOI] [PubMed] [Google Scholar]
- Tso W. W., Adler J. Negative chemotaxis in Escherichia coli. J Bacteriol. 1974 May;118(2):560–576. doi: 10.1128/jb.118.2.560-576.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAITUZIS Z., DOETSCH R. N. FLAGELLA OF SALMONELLA TYPHIMURIUM SPHEROPLASTS. J Bacteriol. 1965 Jun;89:1586–1593. doi: 10.1128/jb.89.6.1586-1593.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]