Abstract
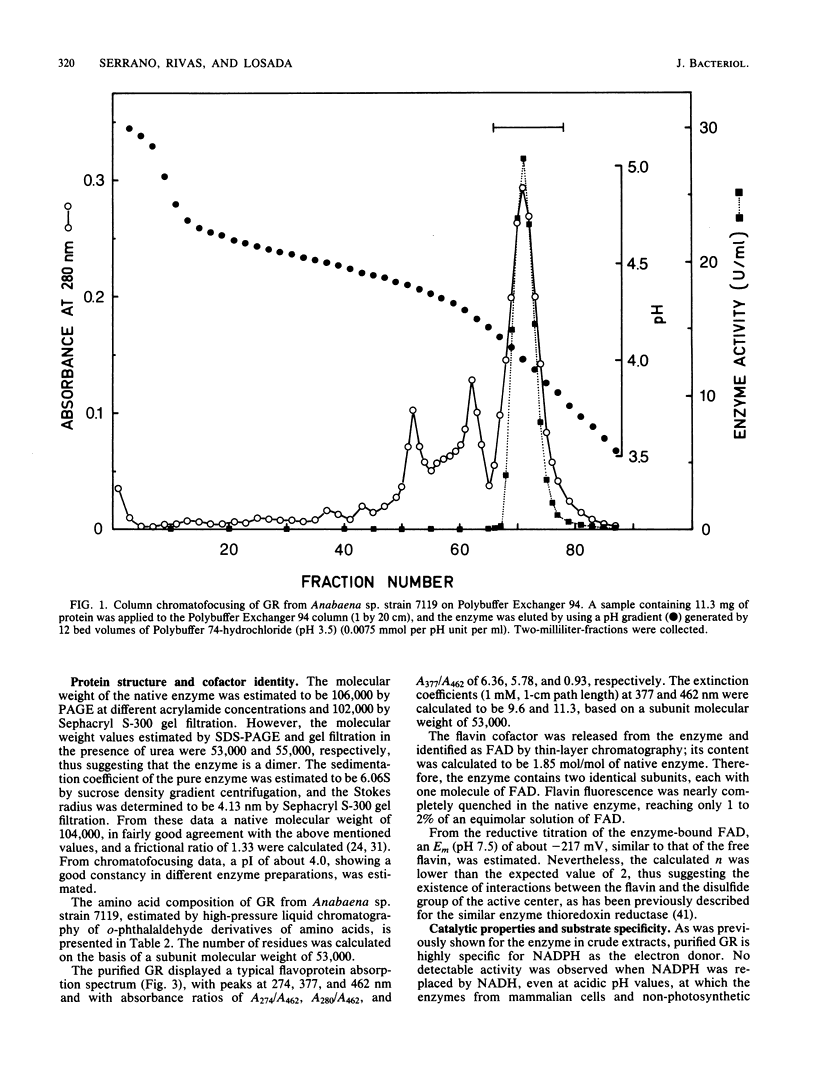
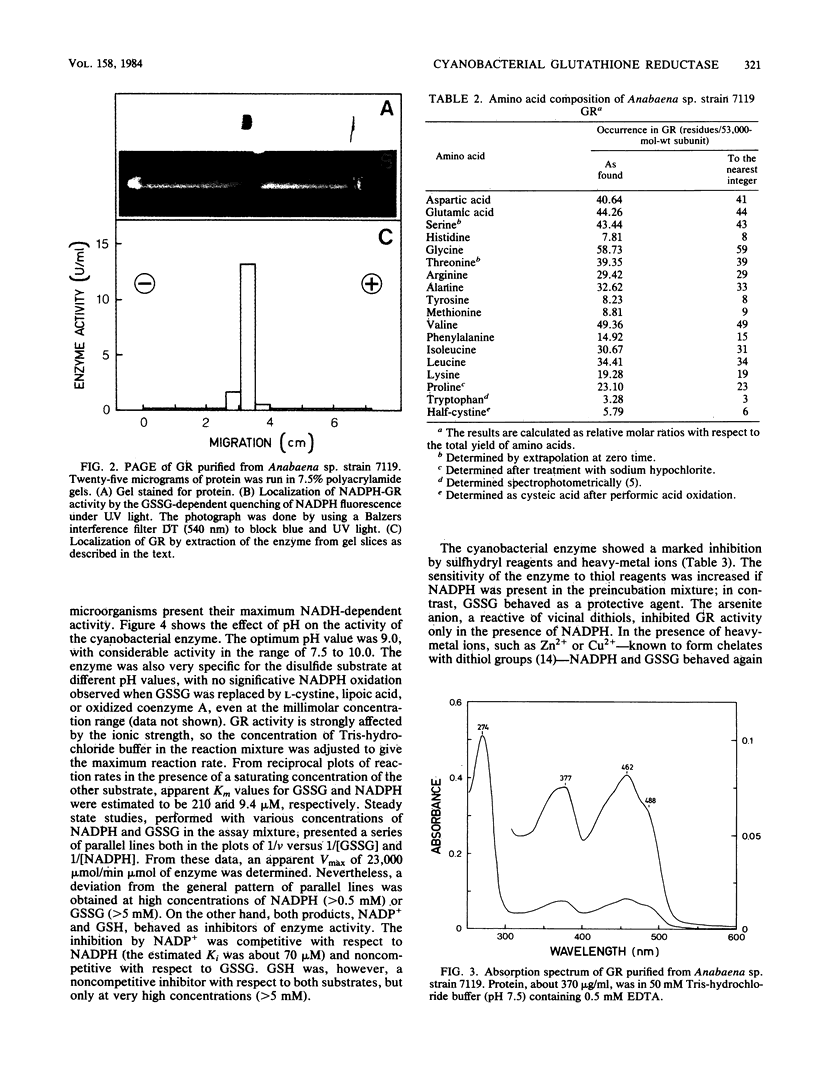
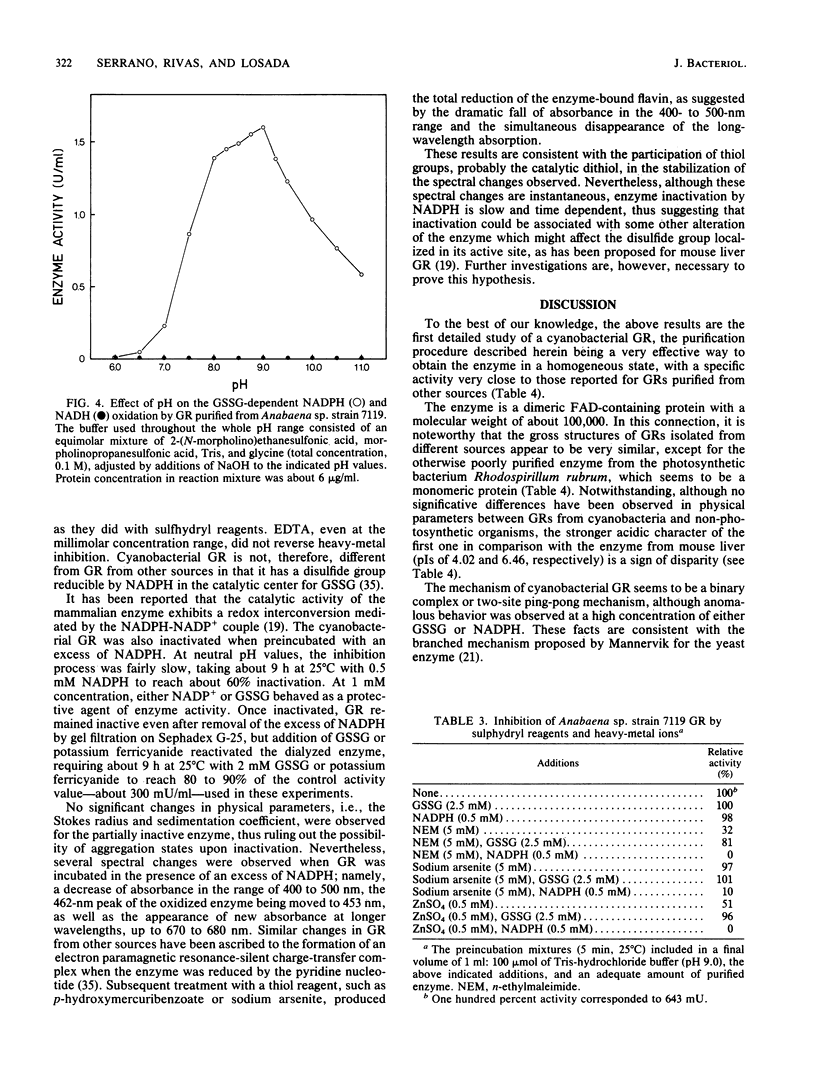
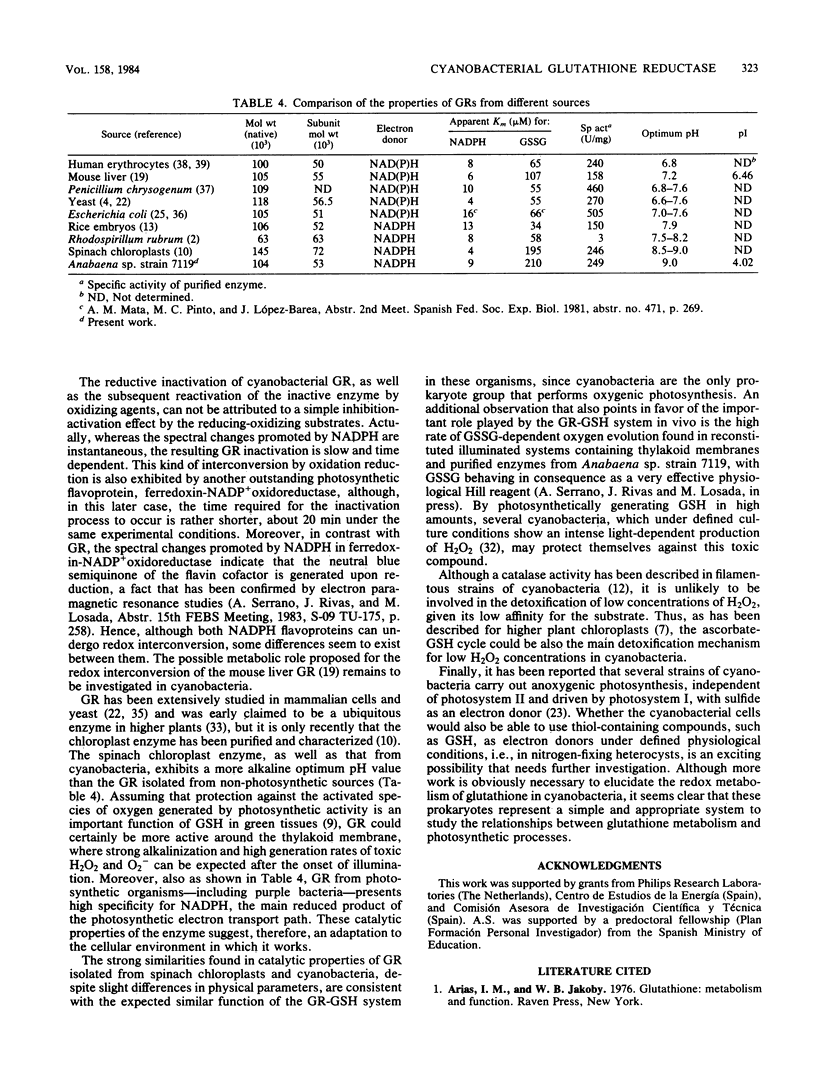
An NADPH-glutathione reductase (EC 1.6.4.2) has been purified 6,000-fold to electrophoretic homogeneity from the filamentous cyanobacterium Anabaena sp. strain 7119. The purified enzyme exhibits a specific activity of 249 U/mg and is characterized by being a dimeric flavin adenine dinucleotide-containing protein with a ratio of absorbance at 280 nm to absorbance at 462 nm of 5.8, a native molecular weight of 104,000, a Stokes radius of 4.13 nm, and a pI of 4.02. The enzyme activity is inhibited by sulfhydryl reagents and heavy-metal ions, especially in the presence of NADPH, with oxidized glutathione behaving as a protective agent. As is the case with the same enzyme from other sources, the kinetic data are consistent with a branched mechanism. Nevertheless, the cyanobacterial enzyme presents three distinctive features with respect to that isolated from non-photosynthetic organisms: (i) absolute specificity for NADPH, (ii) an alkaline optimum pH value of ca. 9.0, and (iii) strong acidic character of the protein, as estimated by column chromatofocusing. The kinetic parameters are very similar to those found for the chloroplast enzyme, but the molecular weight is lower, being comparable to that of non-photosynthetic microorganisms. A protective function, analogous to that assigned to the chloroplast enzyme, is suggested.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boll M. Glutathione reductase from Rhodospirillum rubrum. Arch Mikrobiol. 1969;66(4):374–388. doi: 10.1007/BF00414592. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Giddings T. H., Jr, Wolk C. P., Shomer-Ilan A. Metabolism of sulfur compounds by whole filaments and heterocysts of Anabaena variabilis. J Bacteriol. 1981 Jun;146(3):1067–1074. doi: 10.1128/jb.146.3.1067-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Henry L. E., Gogotov I. N., Hall D. O. Superoxide dismutase and catalase in the protection of the proton-donating systems of nitrogen fixation in the blue-green alga Anabaena cylindrica. Biochem J. 1978 Aug 15;174(2):373–377. doi: 10.1042/bj1740373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOVIN T., CHRAMBACH A., NAUGHTON M. A. AN APPARATUS FOR PREPARATIVE TEMPERATURE-REGULATED POLYACRYLAMIDE GEL ELECTROPHORESIS. Anal Biochem. 1964 Nov;9:351–369. doi: 10.1016/0003-2697(64)90192-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- López-Barea J., Lee C. Y. Mouse-liver glutathione reductase. Purification, kinetics, and regulation. Eur J Biochem. 1979 Aug 1;98(2):487–499. doi: 10.1111/j.1432-1033.1979.tb13210.x. [DOI] [PubMed] [Google Scholar]
- Massey V., Williams C. H., Jr On the reaction mechanism of yeast glutathione reductase. J Biol Chem. 1965 Nov;240(11):4470–4480. [PubMed] [Google Scholar]
- Pigiet V. P., Conley R. R. Purification of thioredoxin, thioredoxin reductase, and glutathione reductase by affinity chromatography. J Biol Chem. 1977 Sep 25;252(18):6367–6372. [PubMed] [Google Scholar]
- Roth M. Fluorescence reaction for amino acids. Anal Chem. 1971 Jun;43(7):880–882. doi: 10.1021/ac60302a020. [DOI] [PubMed] [Google Scholar]
- Schaedle M. Chloroplast glutathione reductase. Plant Physiol. 1977 May;59(5):1011–1012. doi: 10.1104/pp.59.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A., Rivas J. Purification of ferredoxin-NADP+ oxidoreductase from cyanobacteria by affinity chromatography on 2',5'-ADP-sepharose 4B. Anal Biochem. 1982 Oct;126(1):109–115. doi: 10.1016/0003-2697(82)90115-4. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Watson D. H., Harvey M. J., Dean P. D. The selective retardation of NADP+-dependent dehydrogenases by immobilized procion red HE-3B. Biochem J. 1978 Aug 1;173(2):591–596. doi: 10.1042/bj1730591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodin T. S., Segel I. H. Isolation and characterization of glutathione reductase from Penicillium chrysogenum. Biochim Biophys Acta. 1968 Aug 27;167(1):64–77. doi: 10.1016/0005-2744(68)90277-5. [DOI] [PubMed] [Google Scholar]
- Worthington D. J., Rosemeyer M. A. Glutathione reductase from human erythrocytes. Catalytic properties and aggregation. Eur J Biochem. 1976 Aug 1;67(1):231–238. doi: 10.1111/j.1432-1033.1976.tb10654.x. [DOI] [PubMed] [Google Scholar]
- Worthington D. J., Rosemeyer M. A. Glutathione reductase from human erythrocytes. Molecular weight, subunit composition and aggregation properties. Eur J Biochem. 1975 Dec 15;60(2):459–466. doi: 10.1111/j.1432-1033.1975.tb21024.x. [DOI] [PubMed] [Google Scholar]
- Yocum R. R., Blumberg P. M., Strominger J. L. Purification and characterization of the thermophilic D-alanine carboxypeptidase from membranes of Bacillus stearothermophilus. J Biol Chem. 1974 Aug 10;249(15):4863–4871. [PubMed] [Google Scholar]
- Zanetti G., Williams C. H., Jr Characterization of the active center of thioredoxin reductase. J Biol Chem. 1967 Nov 25;242(22):5232–5236. [PubMed] [Google Scholar]




