Abstract
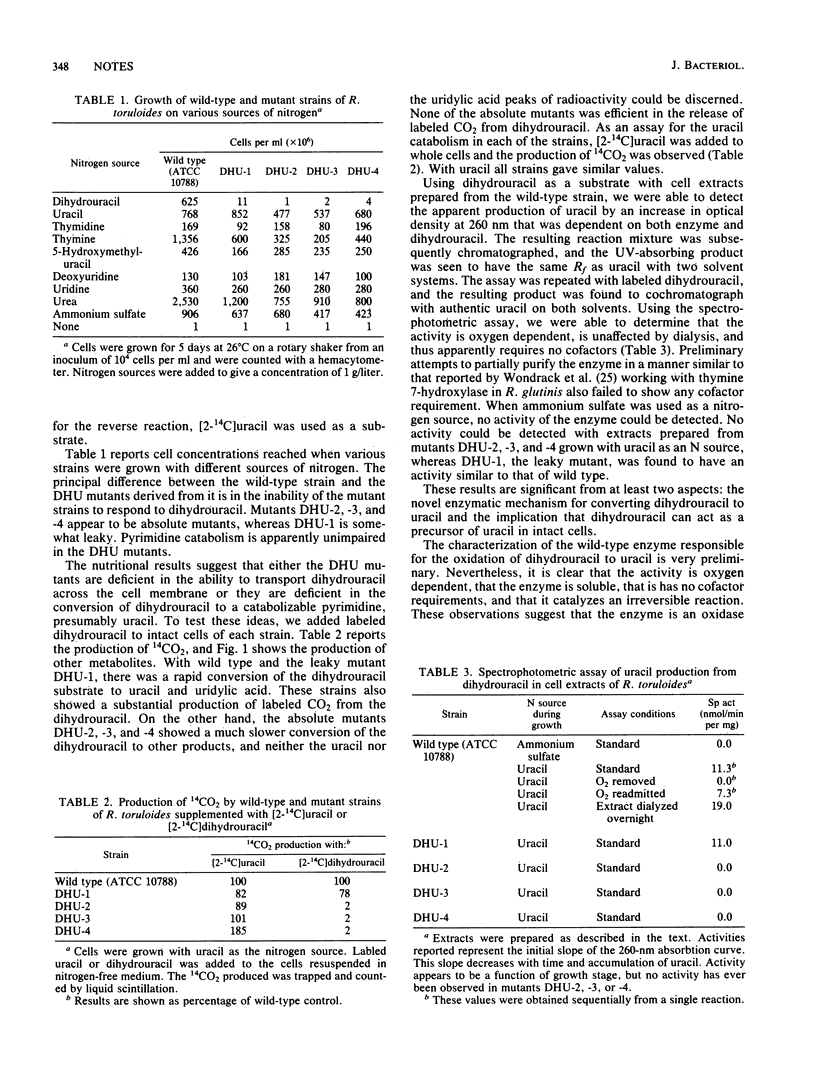
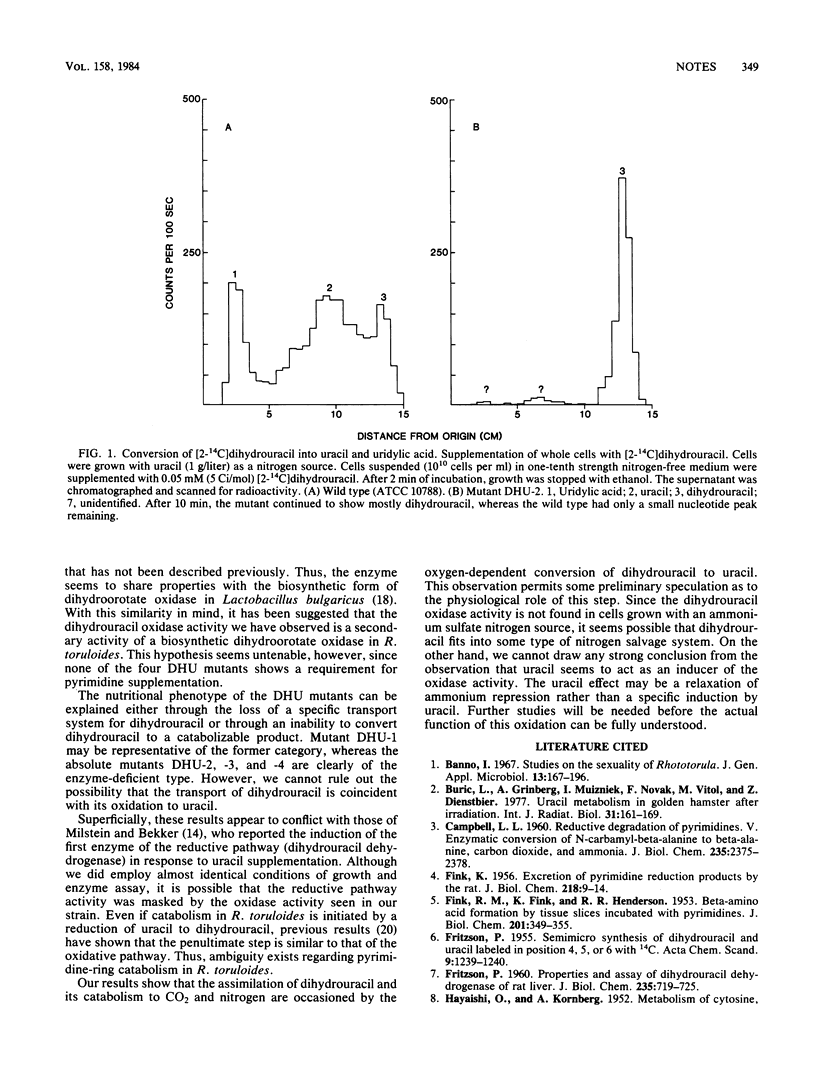
Previous studies, including those done with a similar species, have indicated that dihydrouracil is formed by the breakdown of uracil and is degraded into N-carbamyl-beta-alanine. (Fink et al., J. Biol. Chem. 201:349-355, 1953; S. R. Vilks and M. Y. Vitols, Mikrobiologiya 42:567-583, 1973; O. A. Milstein and M. L. Bekker, J. Bacteriol. 127:1-6, 1976). In the present work the conversion of dihydrouracil to uracil is studied in Rhodosporidium toruloides, and the growth characteristics of mutants that have lost the ability to use dihydrouracil as a source of nitrogen are examined. It is concluded that dihydrouracil must be converted to uracil before catabolism of the pyrimidine ring can take place.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buric L., Grinberg A., Muizniek I., Novák F., Vitol M., Dienstbier Z. Uracil metabolism in golden hamster after irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1977 Feb;31(2):161–169. doi: 10.1080/09553007714550181. [DOI] [PubMed] [Google Scholar]
- CAMPBELL L. L. Reductive degradation of pyrimidines. 5. Enzymatic conversion of N-carbamyl-beta-alanine to beta-alanine, carbon dioxide, and ammonia. J Biol Chem. 1960 Aug;235:2375–2378. [PubMed] [Google Scholar]
- FINK K. Excretion of pyrimidine reduction products by the rat. J Biol Chem. 1956 Jan;218(1):9–14. [PubMed] [Google Scholar]
- FINK R. M., FINK K., HENDERSON R. B. beta-amino acid formation by tissue slices incubated with pyrimidines. J Biol Chem. 1953 Mar;201(1):349–355. [PubMed] [Google Scholar]
- FRITZSON P. Properties and assay of dihydrouracil dehydrogenase of rat liver. J Biol Chem. 1960 Mar;235:719–725. [PubMed] [Google Scholar]
- Hilton M. G. The metabolism of pyrimidines by proteolytic clostridia. Arch Microbiol. 1975;102(2):145–149. doi: 10.1007/BF00428359. [DOI] [PubMed] [Google Scholar]
- Krämer J., Kaltwasser H. Verwertung von Pyrimidinderivaten durch Hydrogenomonas facilis. II. Abbau von Thymin und Uracil durch Wildstamm und Mutanten. Arch Mikrobiol. 1969;69(2):138–148. [PubMed] [Google Scholar]
- LARA F. J. S. On the decomposition of pyrimidines by bacteria. I. Studies by means of the technique of simultaneous adaptation. J Bacteriol. 1952 Aug;64(2):271–277. doi: 10.1128/jb.64.2.271-277.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein O. A., Bekker M. L. Utilization of exogenous pyrimidines as a source of nitrogen by cells of the yeast Rhodotorula glutinis. J Bacteriol. 1976 Jul;127(1):1–6. doi: 10.1128/jb.127.1.1-6.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piret M. C., Crokaert R., Christophe J. Le catabolisme réductif de l'uracile chez Torulopsis utilis. Arch Int Physiol Biochim. 1964 Mar;72(2):256–266. doi: 10.3109/13813456409058971. [DOI] [PubMed] [Google Scholar]
- RUTMAN R. J., CANTAROW A., PASCHKIS K. E. The catabolism of uracil in vivo and in vitro. J Biol Chem. 1954 Sep;210(1):321–329. [PubMed] [Google Scholar]
- Taylor M. L., Taylor W. H., Eames D. F., Taylor C. D. Biosynthetic dihydroorotate dehydrogenase from Lactobacillus bulgaricus. J Bacteriol. 1971 Mar;105(3):1015–1027. doi: 10.1128/jb.105.3.1015-1027.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites W. M., Davis C. H., Wallis-Biggart N., Wondrack L. M., Abbott M. T. Urea: obligate intermediate of pyrimidine-ring catabolism in Rhodosporidium toruloides. J Bacteriol. 1979 Mar;137(3):1145–1150. doi: 10.1128/jb.137.3.1145-1150.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites W. M. Device for the comparison of replica plates. Appl Microbiol. 1968 Jun;16(6):956–957. doi: 10.1128/am.16.6.956-957.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. S., Axelrod B. Catabolism of Pyrimidines in Rape Seedlings. Plant Physiol. 1965 Jan;40(1):39–44. doi: 10.1104/pp.40.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilks S. R., Vitols M. Ia. Usvoenie i katabolizm 5-metiltsitozina i timina drozhzhami Rhodotorula glutinis (Fres.) Harrison. Mikrobiologiia. 1973 Jul-Aug;42(4):576–582. [PubMed] [Google Scholar]
- Vogels G. D., Van der Drift C. Degradation of purines and pyrimidines by microorganisms. Bacteriol Rev. 1976 Jun;40(2):403–468. doi: 10.1128/br.40.2.403-468.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODWARD V. W., MUNKRES K. D., SUYAMA Y. Uracil metabolism in Neurospora crassa. Experientia. 1957 Dec 15;13(12):484–486. doi: 10.1007/BF02159410. [DOI] [PubMed] [Google Scholar]
- Wondrack L. M., Hsu C. A., Abbott M. T. Thymine 7-hydroxylase and pyrimidine deoxyribonucleoside 2' -hydroxylase activities in Rhodotorula glutinis. J Biol Chem. 1978 Sep 25;253(18):6511–6515. [PubMed] [Google Scholar]


