Abstract
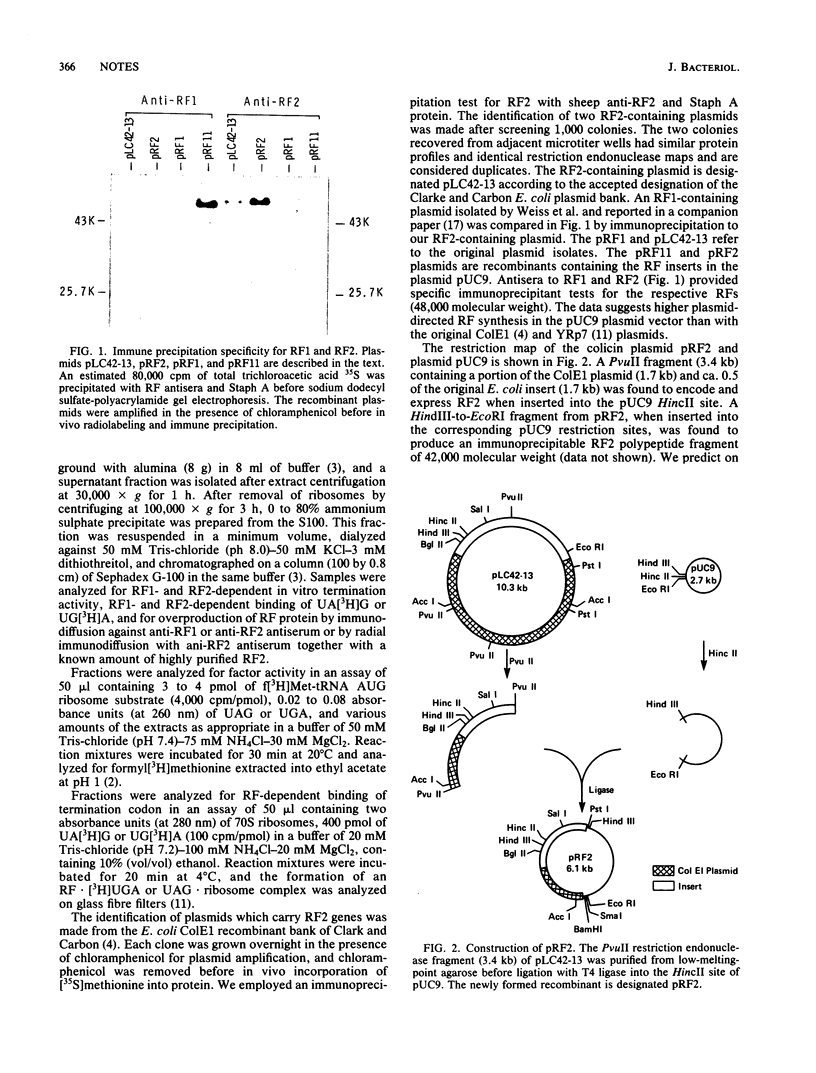
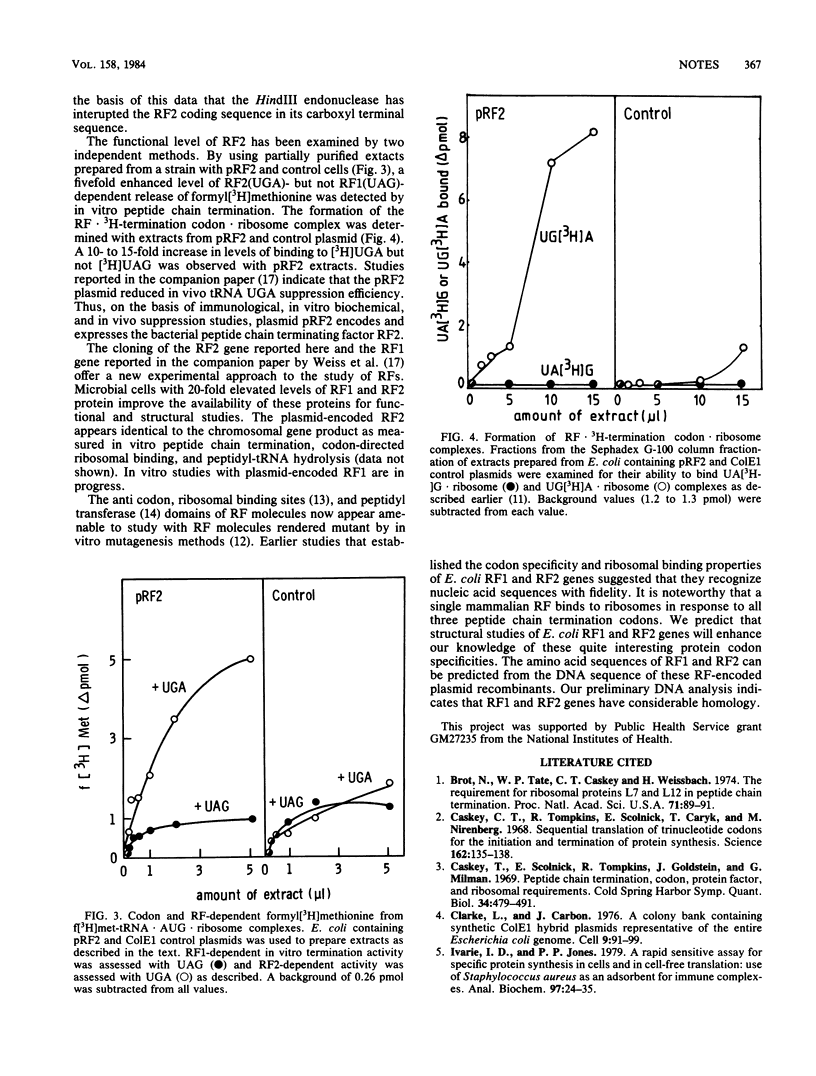
The protein release factor 2 (RF2) participates in Escherichia coli polypeptide chain termination with codon specificity (UAA or UGA). A colicin E1 recombinant identified in the Carbon and Clarke E. coli bank contains the protein release factor 2 gene. A 1.7-kilobase E. coli fragment has been subcloned into the plasmid pUC9 vector. Bacterial cells, containing the plasmid recombinant, produce elevated levels of protein release factor 2 as detected by an immune precipitation assay and in vitro measurement of UGA-directed peptide chain termination and [3H]UGA codon recognition.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caskey C. T., Tompkins R., Scolnick E., Caryk T., Nirenberg M. Sequential translation of trinucleotide codons for the initiation and termination of protein synthesis. Science. 1968 Oct 4;162(3849):135–138. doi: 10.1126/science.162.3849.135. [DOI] [PubMed] [Google Scholar]
- Caskey T., Scolnick E., Tompkins R., Goldstein J., Milman G. Peptide chain termination, codon, protein factor, and ribosomal requirements. Cold Spring Harb Symp Quant Biol. 1969;34:479–488. doi: 10.1101/sqb.1969.034.01.054. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Jones P. P. A rapid sensitive assay for specific protein synthesis in cells and in cell-free translations: use of Staphylococcus aureus as an adsorbent for immune complexes. Anal Biochem. 1979 Aug;97(1):24–35. doi: 10.1016/0003-2697(79)90322-1. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Wirth R., Smith M. W., Van Bogelen R. Selective synthesis of plasmid-coded proteins by Escherichia coli during recovery from chloramphenicol treatment. J Bacteriol. 1980 Jul;143(1):535–537. doi: 10.1128/jb.143.1.535-537.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O., Rössner E. Synthesis of a chemically reactive analog of the nonsense codon U-G-A. Its reaction with ribosomes of Escherichia coli. Hoppe Seylers Z Physiol Chem. 1975 Aug;356(8):1297–1304. doi: 10.1515/bchm2.1975.356.2.1297. [DOI] [PubMed] [Google Scholar]
- Ratliff J. C., Caskey C. T. Immunologic evidence for structural homology between the release factors of Escherichia coli. Arch Biochem Biophys. 1977 Jun;181(2):671–677. doi: 10.1016/0003-9861(77)90273-9. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Caskey C. T. Peptide chain termination. V. The role of release factors in mRNA terminator codon recognition. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1235–1241. doi: 10.1073/pnas.64.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E., Tompkins R., Caskey T., Nirenberg M. Release factors differing in specificity for terminator codons. Proc Natl Acad Sci U S A. 1968 Oct;61(2):768–774. doi: 10.1073/pnas.61.2.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., DiMaio D., Nathans D. Directed mutagenesis. Annu Rev Genet. 1981;15:265–294. doi: 10.1146/annurev.ge.15.120181.001405. [DOI] [PubMed] [Google Scholar]
- Smrt J., Kemper W., Caskey T., Nirenberg M. Template activity of modified terminator codons. J Biol Chem. 1970 May 25;245(10):2753–2757. [PubMed] [Google Scholar]
- Stöffler G., Tate W. P., Caskey C. T. Ribosomal proteins cross-linked to peptide chain termination release factor 2. J Biol Chem. 1982 Apr 25;257(8):4203–4206. [PubMed] [Google Scholar]
- Tompkins R. K., Scolnick E. M., Caskey C. T. Peptide chain termination. VII. The ribosomal and release factor requirements for peptide release. Proc Natl Acad Sci U S A. 1970 Mar;65(3):702–708. doi: 10.1073/pnas.65.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weiss R. B., Murphy J. P., Gallant J. A. Genetic screen for cloned release factor genes. J Bacteriol. 1984 Apr;158(1):362–364. doi: 10.1128/jb.158.1.362-364.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]




