Abstract
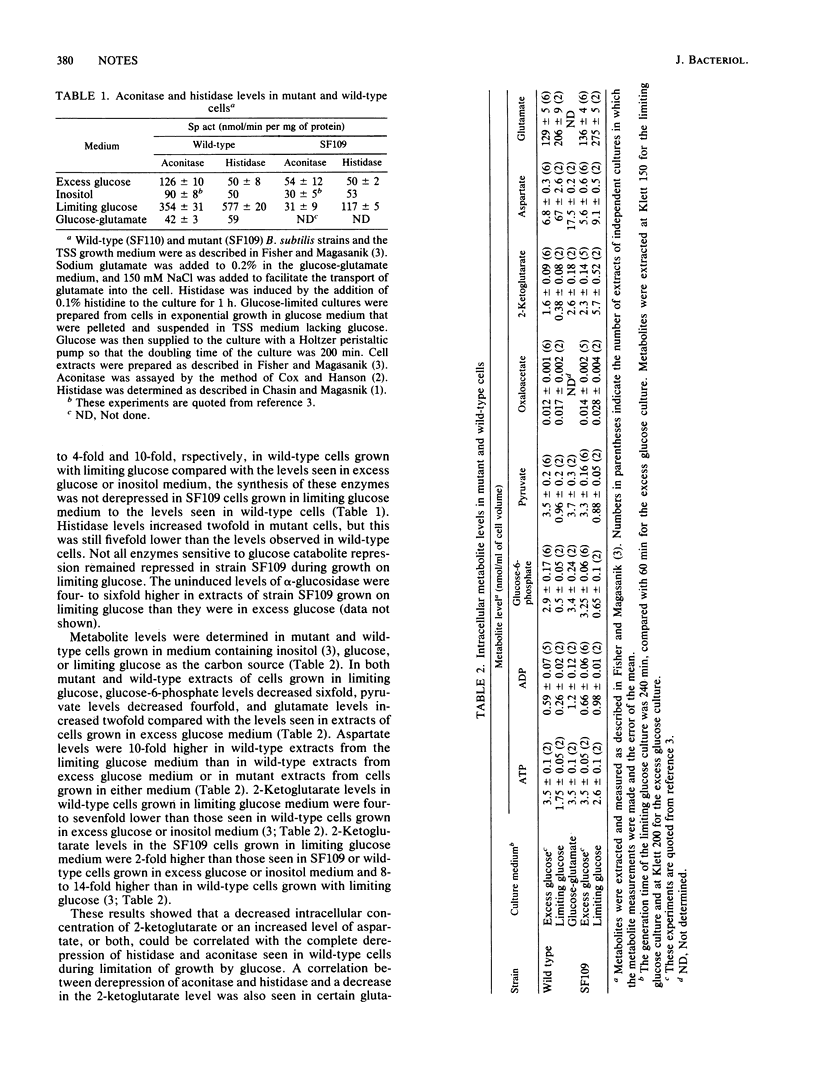
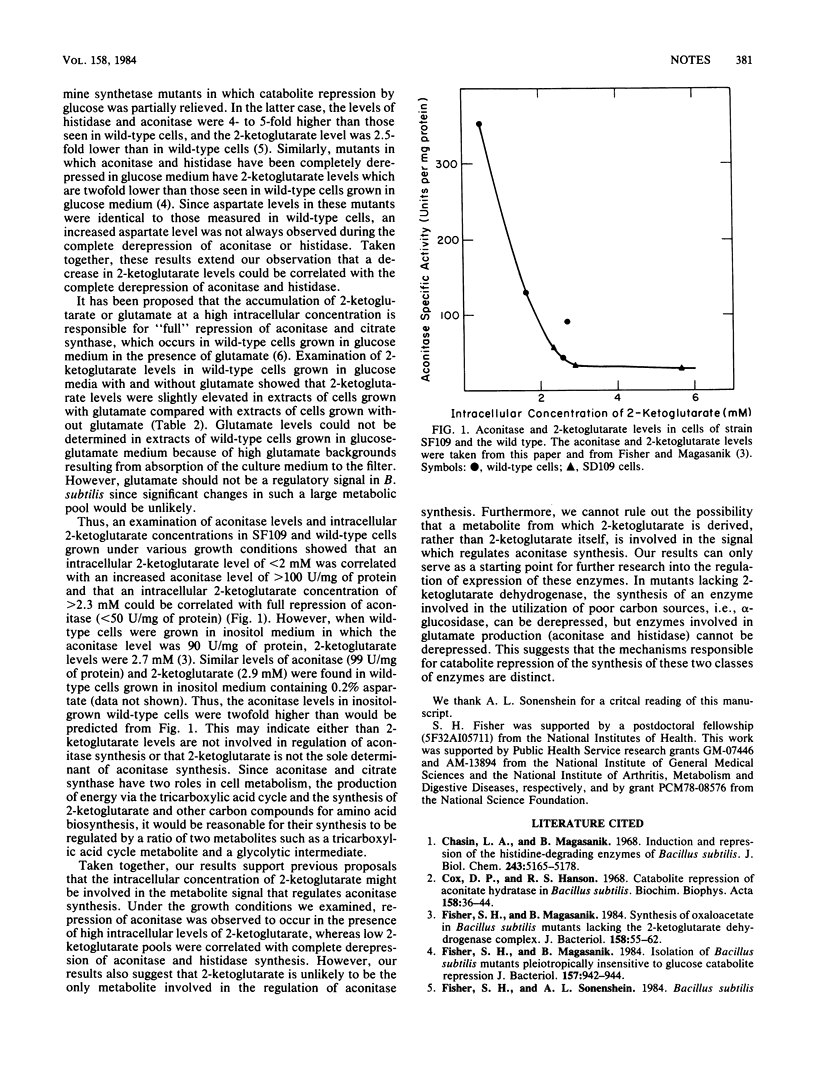
In contrast to wild-type cells, the Bacillus subtilis mutant SF109 that lacks the active 2-ketoglutarate dehydrogenase enzymatic complex is unable to increase the specific activity of two enzymes subject to glucose catabolite repression, aconitase and histidase, during limitation of growth by glucose. Examination of the intracellular metabolite pools in the mutant and wild-type cells grown in excess and limiting glucose medium showed that the complete derepression of aconitase and histidase could be correlated with the decrease in the intracellular concentration of 2-ketoglutarate. The complete repression of aconitase that occurred in wild-type and mutant cells could be correlated with a high intracellular concentration of 2-ketoglutarate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chasin L. A., Magasanik B. Induction and repression of the histidine-degrading enzymes of Bacillus subtilis. J Biol Chem. 1968 Oct 10;243(19):5165–5178. [PubMed] [Google Scholar]
- Cox D. P., Hanson R. S. Catabolite repression of aconitate hydratase in Bacillus subtilis. Biochim Biophys Acta. 1968 Apr 16;158(1):36–44. doi: 10.1016/0304-4165(68)90069-x. [DOI] [PubMed] [Google Scholar]
- Fisher S. H., Magasanik B. Isolation of Bacillus subtilis mutants pleiotropically insensitive to glucose catabolite repression. J Bacteriol. 1984 Mar;157(3):942–944. doi: 10.1128/jb.157.3.942-944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. H., Magasanik B. Synthesis of oxaloacetate in Bacillus subtilis mutants lacking the 2-ketoglutarate dehydrogenase enzymatic complex. J Bacteriol. 1984 Apr;158(1):55–62. doi: 10.1128/jb.158.1.55-62.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechtner V. R., Hanson R. S. Coarse and fine control of citrate synthase from Bacillus subtilis. Biochim Biophys Acta. 1969 Jul 30;184(2):252–262. doi: 10.1016/0304-4165(69)90027-0. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P. The regulation of aconitase and isocitrate dehydrogenase in sporulation mutants of Bacillus subtilis. Biochim Biophys Acta. 1970 Nov 24;222(2):290–298. doi: 10.1016/0304-4165(70)90116-9. [DOI] [PubMed] [Google Scholar]
- Hanson R. S., Cox D. P. Effect of different nutritional conditions on the synthesis of tricarboxylic acid cycle enzymes. J Bacteriol. 1967 Jun;93(6):1777–1787. doi: 10.1128/jb.93.6.1777-1787.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohné M. Regulation of aconitase synthesis in Bacillus subtilis: induction, feedback repression, and catabolite repression. J Bacteriol. 1974 Mar;117(3):1295–1305. doi: 10.1128/jb.117.3.1295-1305.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohné M., Rutberg B., Hoch J. A. Genetic and biochemical characterization of mutants of Bacillus subtilis defective in succinate dehydrogenase. J Bacteriol. 1973 Sep;115(3):738–745. doi: 10.1128/jb.115.3.738-745.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg B., Hoch J. A. Citric acid cycle: gene-enzyme relationships in Bacillus subtilis. J Bacteriol. 1970 Nov;104(2):826–833. doi: 10.1128/jb.104.2.826-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



