Abstract
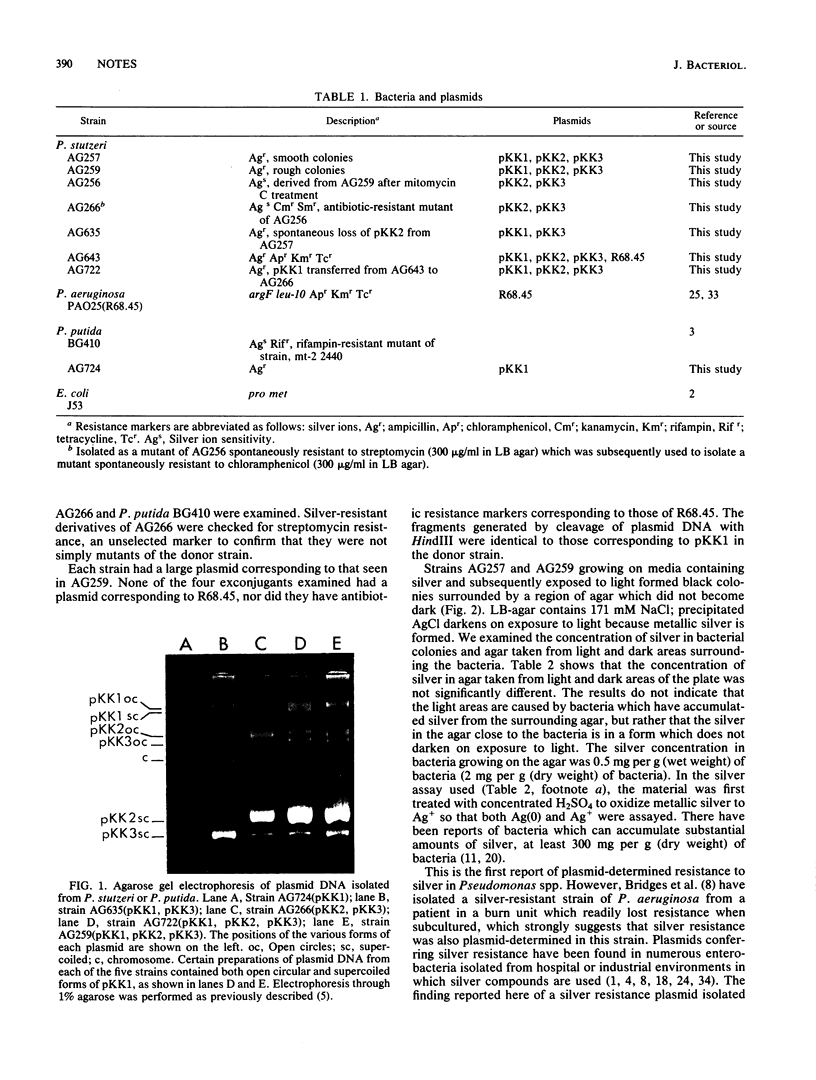
A silver-resistant strain of Pseudomonas stutzeri was isolated from a silver mine. It harbored three plasmids, the largest of which (pKK1; molecular weight, 49.4 X 10(6)) specified silver resistance. Plasmid pKK1 was apparently nonconjugative but could be transferred to Pseudomonas putida by mobilization with plasmid R68.45.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annear D. I., Mee B. J., Bailey M. Instability and linkage of silver resistance, lactose fermentation, and colony structure in Enterobacter cloacae from burn wounds. J Clin Pathol. 1976 May;29(5):441–443. doi: 10.1136/jcp.29.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Binns M. M., Davies D. L., Hardy K. G. Cloned fragments of the plasmid ColV,I-K94 specifying virulence and serum resistance. Nature. 1979 Jun 28;279(5716):778–781. doi: 10.1038/279778a0. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg P. D., Rainnie D. J. The effect of silver ions on the respiratory chain of Escherichia coli. Can J Microbiol. 1974 Jun;20(6):883–889. doi: 10.1139/m74-135. [DOI] [PubMed] [Google Scholar]
- Bridges K., Kidson A., Lowbury E. J., Wilkins M. D. Gentamicin- and silver-resistant pseudomonas in a burns unit. Br Med J. 1979 Feb 17;1(6161):446–449. doi: 10.1136/bmj.1.6161.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPPELL J. B., GREVILLE G. D. Effect of silver ions on mitochondrial adenosine triphosphatase. Nature. 1954 Nov 13;174(4437):930–931. doi: 10.1038/174930b0. [DOI] [PubMed] [Google Scholar]
- Charley R. C., Bull A. T. Bioaccumulation of silver by a multispecies community of bacteria. Arch Microbiol. 1979;123(3):239–244. doi: 10.1007/BF00406656. [DOI] [PubMed] [Google Scholar]
- Downing R. G., Duggleby C. J., Villems R., Broda P. An endonuclease cleavage map of the plasmid pWWO-8, a derivative of the TOL plasmid of Pseudomonas putida mt-2. Mol Gen Genet. 1979 Jan 5;168(1):97–99. doi: 10.1007/BF00267938. [DOI] [PubMed] [Google Scholar]
- Foster T. J., Nakahara H., Weiss A. A., Silver S. Transposon A-generated mutations in the mercuric resistance genes of plasmid R100-1. J Bacteriol. 1979 Oct;140(1):167–181. doi: 10.1128/jb.140.1.167-181.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh G. L., Moellering R. C., Hopkins C. C., Swartz M. N. Salmonella typhimurium resistant to silver nitrate, chloramphenicol, and ampicillin. Lancet. 1975 Feb 1;1(7901):235–240. doi: 10.1016/s0140-6736(75)91138-1. [DOI] [PubMed] [Google Scholar]
- Nakazawa T., Inouye S., Nakazawa A. Physical and functional mapping of RP4-TOL plasmid recombinants: analysis of insertion and deletion mutants. J Bacteriol. 1980 Oct;144(1):222–231. doi: 10.1128/jb.144.1.222-231.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie D. J., Bragg P. D. The effect of iron deficiency on respiration and energy-coupling in Escherichia coli. J Gen Microbiol. 1973 Aug;77(2):339–349. doi: 10.1099/00221287-77-2-339. [DOI] [PubMed] [Google Scholar]
- Rayman M. K., Lo T. C., Sanwal B. D. Transport of succinate in Escherichia coli. II. Characteristics of uptake and energy coupling with transport in membrane preparations. J Biol Chem. 1972 Oct 10;247(19):6332–6339. [PubMed] [Google Scholar]
- Rosenkranz H. S., Carr H. S. Silver sulfadiazine: effect on the growth and metabolism of bacteria. Antimicrob Agents Chemother. 1972 Nov;2(5):367–372. doi: 10.1128/aac.2.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz H. S., Coward J. E., Wlodkowski T. J., Carr H. S. Properties of silver sulfadiazine-resistant Enterobacter cloacae. Antimicrob Agents Chemother. 1974 Feb;5(2):199–201. doi: 10.1128/aac.5.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle P. L., Matsumoto H., Holloway B. W. Genetic circularity of the Pseudomonas aeruginosa PAO chromosome. J Bacteriol. 1981 Jan;145(1):145–155. doi: 10.1128/jb.145.1.145-155.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottel J. L. The mercuric and organomercurial detoxifying enzymes from a plasmid-bearing strain of Escherichia coli. J Biol Chem. 1978 Jun 25;253(12):4341–4349. [PubMed] [Google Scholar]
- Schottel J., Mandal A., Clark D., Silver S., Hedges R. W. Volatilisation of mercury and organomercurials determined by inducible R-factor systems in enteric bacteria. Nature. 1974 Sep 27;251(5473):335–337. doi: 10.1038/251335a0. [DOI] [PubMed] [Google Scholar]
- Schreurs W. J., Rosenberg H. Effect of silver ions on transport and retention of phosphate by Escherichia coli. J Bacteriol. 1982 Oct;152(1):7–13. doi: 10.1128/jb.152.1.7-13.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H. R factors mediate resistance to mercury, nickel, and cobalt. Science. 1967 May 26;156(3778):1114–1116. doi: 10.1126/science.156.3778.1114. [DOI] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Stanisich V. A., Holloway B. W. Chromosome transfer in Pseudomonas aeruginosa mediated by R factors. Genet Res. 1971 Apr;17(2):169–172. doi: 10.1017/s0016672300012179. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Silver S. Mercury resistance in a plasmid-bearing strain of Escherichia coli. J Bacteriol. 1972 Dec;112(3):1228–1236. doi: 10.1128/jb.112.3.1228-1236.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Sugarman L. I. Cell-free mercury(II)-reducing activity in a plasmid-bearing strain of Escherichia coli. J Bacteriol. 1974 Jul;119(1):242–249. doi: 10.1128/jb.119.1.242-249.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN NIEL C. B., ALLEN M. B. A note on Pseudomonas stutzeri. J Bacteriol. 1952 Sep;64(3):413–422. doi: 10.1128/jb.64.3.413-422.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]