Abstract
Cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4) is an immunoregulatory molecule expressed by activated T cells and resting CD4+CD25+ T cells. In patients with advanced melanoma, our group reported that administration of anti-CTLA-4 antibody mediated objective cancer regression in 13% of patients. This study also established that the blockade of CTLA-4 was associated with grade III/IV autoimmune manifestations that included dermatitis, enterocolitis, hepatitis, uveitis, and a single case of hypophysitis. Since this initial report, 7 additional patients with anti-CTLA-4 antibody–induced autoimmune hypophysitis have been accumulated. The characteristics, clinical course, laboratory values, radiographic findings, and treatment of these 8 patients are the focus of this report.
Keywords: anti-CTLA-4 antibody, autoimmunity, autoimmune hypophysitis, melanoma, renal cell cancer, immunotherapy
Cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) is an immunoregulatory molecule expressed by activated T cells and a subset of regulatory T cells. The state of activation of a lymphocyte depends on the simultaneous engagement of costimulatory receptors as well as on the engagement of its T-cell receptor, which induces interleukin (IL)-2 production, proliferation, and differentiation of the T cell. Engagement of B7 molecules on the surface of antigen-presenting cells with CD28 on the surface of T cells activates the T cell. In contrast, reaction with CTLA-4 on the T cell inhibits activation.
In patients with advanced melanoma, we have reported that administration of anti-CTLA-4 antibody mediated objective cancer regression in 13% of patients.1 This study as well as the treatment of additional patients established that the blockade of CTLA-4 was associated with grade III/IV autoimmune manifestations in 25% of patients (14 of 56 patients, unpublished data). These manifestations included dermatitis, enterocolitis, hepatitis, uveitis, and hypophysitis. Since our initial report,2 we have accumulated 7 additional patients with anti-CTLA-4 antibody–induced autoimmune hypophysitis. These 8 patients are the focus of this report.
PATIENTS
As of January 1, 2005, 163 patients with advanced melanoma or renal cell cancer have been enrolled and evaluated on 3 separate institution review board (IRB)–approved clinical trials for infusion of human monoclonal anti-CTLA-4 antibody (MDX-010; Medarex) at the Surgery Branch, National Cancer Institute (NCI). All patients had a staging evaluation that included physical examination; blood hematology and chemistry tests, computed tomography (CT) scans of the chest, abdomen, and pelvis; and brain magnetic resonance imaging (MRI). One hundred thirteen patients with metastatic melanoma (79 men and 34 women) and 50 patients with metastatic renal cell cancer (39 men and 11 women) have been evaluated. Eight of these patients (4.9%) have developed autoimmune hypophysitis while receiving anti-CTLA-4 antibody. Table 1 details the characteristics of these patients.
TABLE 1.
Patient Characteristics and Clinical Response
| Patient | Age (y)/Sex | Diagnosis | Disease Sites | Prior IL-2 | No. Doses | Duration of Treatment (Wk) | Highest Dose Received (mg/kg) | Response* | Response Duration (Mo) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 31/M | Melanoma | Lung | No | 5 | 12 | 9 | NR | — |
| 2 | 56/M | Melanoma | Lung, brain, subcutaneous | No | 5 | 12 | 3 | CR | 26+ |
| 3 | 47/M | RCC | Lung, renal bed, retroperitoneum, liver | Yes | 4 | 9 | 3 | NR | — |
| 4 | 56/M | RCC | Lung, hilum | Yes (LD) | 5 | 12 | 3 | PR | 2 |
| 5 | 61/M | Melanoma | Lung, muscle | Yes | Dose escalate: 6 | 15 | 9 | PR | 4 |
| 6 | 43/M | Melanoma | Adrenal, axilla | Yes | Dose escalate: 7 | 18 | 9 | NR | — |
| 7 | 51/M | Melanoma | Retroperitoneum, muscle, subcutaneous | No | Dose escalate: 7 | 18 | 5 | PR | 5+ |
| 8 | 48/M | Melanoma | Retroperitoneum | Yes | Dose escalate: 9 | 24 | 9 | PR | 4+ |
Responses as of March 1, 2005.
CR indicates complete responder; F, female; LD, low dose; M, male; NR, nonresponder; PR, partial responder; RCC, renal cell carcinoma.
All patients received the anti-CTLA-4 antibody intravenously every 3 weeks. Patients 3 and 4 with renal cell cancer received a dose of 3 mg/kg. Patient 3 received a total of 4 doses, and patient 4 received 5 doses. Patient 2, previously reported in the literature,2 who had melanoma, received anti-CTLA-4 antibody at a dose of 3 mg/kg given in combination with GP100:209–217 (210 m) and GP100:280–288 (288v) peptides emulsified in Incomplete Freund's Adjuvant (IFA) administered every 3 weeks for 5 cycles. The remaining 5 patients, all with melanoma, were treated with anti-CTLA-4 antibody in an intrapatient dose-escalating fashion. Dosing for these patients was started at 3 mg/kg and escalated after 2 doses if an objective tumor response was not obtained. All dosing ceased if grade III/IV toxicity occurred. Patient 1 received 5 doses in total and reached a maximum dose of 9 mg/kg. Patient 5 received 6 doses and reached a maximum dose of 9 mg/kg. Patients 6 and 7 each received 7 doses, patient 6 reached a maximum dose of 5 mg/kg, and patient 7 reached a maximum dose of 9 mg/kg. Patient 8 received 9 doses and reached a maximum dose of 9 mg/kg.
Six (5%) of 113 patients with metastatic melanoma and 2 (4%) of 50 patients with metastatic renal cell cancer developed hypophysitis. Five of these patients had an objective tumor response to anti-CTLA-4 antibody, including 1 patient with a complete response. Five patients had previous IL-2 treatment: 1 with low-dose IL-2 treatment and 4 with high-dose IL-2 therapy. The minimum duration of antibody therapy before the onset of symptoms was 9 weeks (4 doses). All the patients with hypophysitis were male.
CLINICAL FINDINGS
The clinical findings and associated endocrine abnormalities for these patients are presented in Table 2. Symptoms included profound fatigue that interfered with activities of daily life, debilitating headaches that necessitated intravenous narcotics in some cases, memory loss, and loss of libido. Seven of the 8 patients with autoimmune hypophysitis had an increase in the size of the pituitary gland with evidence of suprasellar extension. The eighth patient was found to have an empty sella before enrollment in the protocol.
TABLE 2.
Clinical Symptoms and Radiographic Findings
| Patient No. | Diagnosis | Clinical Presentation | Endocrine Abnormalities | Imaging | Treatment Regimen |
|---|---|---|---|---|---|
| 1 | Melanoma | Fatigue, depression, short-tempered, insomnia, constipation | Hypothyroid with low TSH, low testosterone, hypocortisolism with low corticotropin | Enlarged pituitary with suprasellar extension | Replacement hormones |
| 2 | Melanoma | Personality changes, loss of short-term memory | Hypothyroid with low TSH, low testosterone, hypocortisolism with low corticotropin, hypoprolactinemia | Enlarged pituitary with suprasellar extension | Replacement hormones |
| 3 | RCC | Severe headaches, diarrhea with autoimmune enteritis, fatigue and loss of libido | Hypothyroidism with low TSH, hypocortisolism with low corticotropin, hypoprolactinemia | Enlarged pituitary | High-dose steroids, followed by replacement hormones |
| 4 | RCC | Anxiety, fatigue, impotence, arthritis | Hypothyroidism with low TSH, hypocortisolism with low corticotropin, low testosterone | Empty sella | Replacement hormones |
| 5 | Melanoma | Frontal headaches, fatigue, arthritis in the upper extremities | Hypothyroidism with low TSH, hypocortisolism with low corticotropin, low testosterone | Enlarged pituitary with suprasellar extension | High-dose steroids, followed by replacement hormones |
| 6 | Melanoma | Fatigue limiting daily activities | Hypothyroidism with low TSH, hypocortisolism with low corticotropin, low testosterone | Enlarged pituitary with suprasellar extension | High-dose steroids, followed by replacement hormones |
| 7 | Melanoma | Severe headaches, mild fatigue | Hypothyroidism with low TSH, hypocortisolism with low corticotropin, low testosterone | Enlarged pituitary with suprasellar extension | High-dose steroids, followed by replacement hormones |
| 8 | Melanoma | Severe fatigue, constipation, arthritis and muscle aches | Hypothyroidism with low TSH, hypocortisolism with low corticotropin | Enlarged pituitary with suprasellar extension | High-dose steroids, followed by replacement hormones |
RCC indicates renal cell carcinoma.
To determine whether pituitary gland enlargement was unique to patients with evidence of hypophysitis, pituitary gland measurements for these patients were compared with measurements of 5 patients on this protocol treated with anti-CTLA-4 antibody who had autoimmune symptoms other than hypophysitis and with 6 patients treated with anti-CTLA-4 antibody who had no autoimmune symptoms. These data are presented in Table 3. The pituitary gland was measured on a sagittal MRI section by recording the longest height of the gland. In our studies, brain MRI scans were performed at the end of each course of therapy or when symptoms developed. Seven of 8 patients with hypophysitis had more than a 60% increase in the size of their pituitary gland, and 5 of these patients had a greater than 100% increase in the size of their pituitary gland (see Table 3). In contrast, 6 patients chosen at random from these protocols without evidence of autoimmune side effects and 5 patients chosen at random with evidence of autoimmune side effects other than hypophysitis demonstrated minimal if any increase in pituitary gland size. Figure 1 illustrates the typical MRI findings associated with hypophysitis.
TABLE 3.
Pituitary Height (mm) in 3 Patient Subsets
| Hypopituitary Patients
|
Anti-CTLA-4 Treatment: No Autoimmunity
|
Anti-CTLA-4 Treatment: Autoimmunity
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Pre-Tx (mm) | Max (mm) | Change (%) | Pre-Tx (mm) | Maximum (mm) | Change (%) | Pre-Tx (mm) | Maximum (mm) | Change (%) |
| 1 | 5.3 | 10.7 | 102 | 5.3 | 5.9 | 11 | 5.6 | 4.8 | −14 |
| 2 | 3.4 | 11.5 | 238 | 6 | 5.6 | −7 | 7.7 | 7.7 | 0 |
| 3 | 4.8 | 7.7 | 60 | 7.8 | 8.7 | 12 | 6.4 | 7.9 | 23 |
| 4 | 0 | 0 | 0 | 5.4 | 3.1 | −43 | 6 | 6.5 | 8 |
| 5 | 6 | 10.2 | 70 | 6.6 | 7.6 | 15 | 7 | 7 | 0 |
| 6 | 5.5 | 11.5 | 109 | 6.6 | 5.5 | −17 | − | − | − |
| 7 | 4.5 | 11.8 | 162 | − | − | − | − | − | − |
| 8 | 4.1 | 11.4 | 178 | − | − | − | − | − | − |
Pre-Tx measurement taken before anti-CTLA-4 antibody treatment.
Max measurement taken at the maximal height of the pituitary gland while on treatment.
Pre-Tx indicates pretreatment.
FIGURE 1.
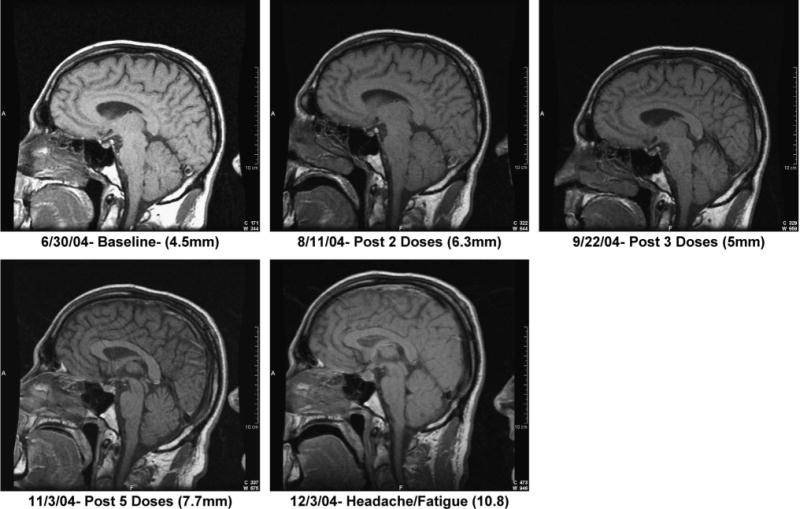
Sagittal MRI section from patient 7 before anti-CTLA-4 antibody treatment and at various intervals during treatment, including the time of clinical symptom onset. Pituitary height is in parentheses.
Table 4 lists the serum levels of cortisol, corticotropin, free thyroxine, thyroid-stimulating hormone (TSH), and total testosterone for each patient with hypophysitis. Before anti-CTLA-4 antibody infusion, all patients had normal endocrine function. Levels obtained when patients were symptomatic document clear evidence of panhypopituitarism. All patients had low levels of cortisol, and 7 of these patients showed inappropriately low levels of corticotropin. In addition, TSH levels were documented to be inappropriately low for all 8 patients, given the corresponding levels of free thyroxine. Finally, 7 of these men developed low serum testosterone. Two of 8 patients had elevated prolactin levels.
TABLE 4.
Pretreatment Laboratory Values and Posttreatment Laboratory Values Taken After Diagnosis of Hypopituitarism and Before Steroid Replacement
| Hormone* | Patient No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|
| Cortisol (5.0–25.0 μg/dL) | Pre: | 7.8 | 22.4 | 14.8 | 7.3 | 16.9 | 14.1 | 22.2 | 13.7 |
| Post: | <0.1 | <0.1 | <0.1 | <0.1 | <1.0 | <1.0 | <1.0 | <0.1 | |
| Corticotropin (9.0–52.0 pg/mL) | Pre: | † | |||||||
| Post: | 3.3 | 3.8 | 3.6 | <0.1 | 5.3 | 2.5 | 45.3 | 8 | |
| TSH (0.4–4.0 μIU/mL) | Pre: | 1.2 | 2.08 | 2.82 | 0.57 | 0.66 | 2.45 | 1.05 | |
| Post: | 0.41 | 0.18 | <0.02 | 0.91 | <0.02 | 0.18 | 0.1 | 0.25 | |
| Free thyroxine (0.8–1.9 ng/dL) | Pre: | 1 | 1.3 | 0.8 | 1.4 | 1.1 | 0.9 | 1.2 | |
| Post: | 0.5 | 0.4 | 1.3 | 0.4 | 1.1 | 1.2 | 0.7 | 0.8 | |
| Total testosterone (262.0–1593.0 ng/dL) | Pre: | 359 | 286 | 409 | 528 | 487 | 315 | ||
| Post: | 43.8 | 20 | 378 | 92 | <20 | 62.6 | <20.0 | 278 |
Normal ranges are in parentheses.
Precorticotropin levels could not be measured because plasma was not cryopreserved.
CLINICAL COURSE
All 8 patients had resolution of their symptoms once anti-CTLA-4 antibody dosing was discontinued and steroid, thyroid, and testosterone replacement was initiated. Because of the concern that exogenous steroids might reverse tumor regression, the first 3 patients were treated for panhypopituitarism with physiologic doses of steroids, thyroid hormone, and testosterone. An attempt was made to wean patient 2 off hormone replacements approximately 2 years after the diagnosis of hypophysitis. His testosterone replacement was discontinued, and his levothyroxine was transitioned to liothyronine, which was then temporarily discontinued. The patient developed memory loss and fatigue after replacements were stopped. Free T4, TSH, and corticotropin levels were all low at this point. His thyroid and testosterone replacement hormones were reinstituted, with subsequent relief of symptoms.
In an effort to reverse the anti-CTLA-4 antibody–induced panhypopituitarism, we elected to treat subsequent patients with a short course of intravenous high-dose dexamethasone. This strategy was based on our experience that other autoimmune side effects associated with anti-CTLA-4 antibody infusion, such as colitis, have been rapidly and permanently reversed with short courses of dexamethasone. In addition, in patients with objective tumor responses to anti-CTLA-4 antibody, this steroid therapy seemed to have no adverse impact on tumor responses. Consequently, 4 patients were first treated with intravenous dexamethasone (4 mg every 6 hours) for 7 days and then rapidly tapered to maintenance doses of hydrocortisone. These patients were also treated with replacement thyroid hormone and testosterone if indicated. On follow-up, 3 patients treated with this strategy have demonstrated some return of pituitary function. Patient 5 was started on high-dose steroids for 1 week and then rapidly tapered to replacement levels of hydrocortisone. He was also started on replacement levothyroxine and testosterone. The patient returned for repeat laboratory tests after his steroid taper and was instructed to discontinue testosterone replacement a week before his next evaluation. His subsequent evaluation demonstrated that his corticotropin level had increased and his testosterone, TSH, and free T4 levels were all within the normal range. He was instructed to remain on hydrocortisone replacement. He is being closely followed. As of February 2005, all 8 patients remain on hydrocortisone replacement at times from 4 to 26 months after the initiation of symptoms. Patients 1 through 3 remain on full steroid, thyroid, and testosterone replacements. Patient 4 been weaned off testosterone replacement, and his steroid dose has been lowered. Patients 5 and 7 have started to wean their hydrocortisone doses, and patients 5 through 8 are not requiring any thyroid or testosterone replacements at this point.
DISCUSSION
Autoimmune hypophysitis (also known as lymphocytic hypophysitis) is a rare autoimmune disease characterized by infiltration of the pituitary gland by lymphocytes, plasma cells, and macrophages.3 This infiltration causes an impairment of pituitary gland function. Autoimmune hypophysitis typically has a strong female predilection. Women are affected 9 times more frequently than men and at an earlier age. Most patients are affected during late pregnancy or in the postpartum period. Because this disorder is associated with an enlargement of the pituitary gland, patients frequently present with headaches, visual field impairment, and, rarely, diploplia.4 The diagnosis of autoimmune hypophysitis is supported with the correlative findings of pituitary enlargement on MRI. It has been reported that the first and most frequent pituitary hormone to be affected by autoimmune hypophysitis in these patients is corticotropin.5 Prolactin levels, conversely, tend to be elevated in patients with this disorder. This may be attributable to pressure effects on the infundibulum. Hyperprolactinemia affects one third of patients and can cause amenorrhea in woman and sexual dysfunction in men.6
The prognosis of patients with autoimmune hypophysitis is variable. Patients may remain asymptomatic, have spontaneous resolution of their hypophysitis, or, at the opposite end of the spectrum, progress to pituitary fibrosis and panhypopituitarism.
Although there is no consensus regarding therapy, there are several treatment options available. Replacement therapy or isohormonal therapy has been shown to restore some endocrine function in other autoimmune endocrine diseases but has not been tested in autoimmune hypophysitis.7 High-dose methylprednisolone pulse therapy seems to be effective in approximately 30% of patients,8 and positive results have also been reported with cyclosporin A.9
Although autoimmune endocrinopathies such as hyperthyroidism, hypothyroidism, and insulin-dependent diabetes are recognized side effects of immunotherapy, hypophysitis in this setting seems to be rare.10 The clinical evidence strongly suggests that our patients developed autoimmune hypophysitis as a consequence of anti-CTLA-4 antibody infusion. All our patients developed symptoms and laboratory abnormalities consistent with panhypopituitarism. Seven patients showed clear evidence of pituitary gland enlargement, and the patient without gland enlargement had an empty sella at baseline. In addition, exogenous steroid hormone replacement eliminated the associated clinical symptoms and reduced pituitary enlargement on follow-up MRI evaluations. Finally, our group has shown that the administration of multiple doses of anti-CTLA-4 antibody (along with peptide vaccination) in patients with melanoma can be associated with the development of other grade III/IV autoimmune toxicities, including dermatitis, enterocolitis, and hepatitis.1,2 It has also been recognized that the development of autoimmunity correlates with tumor regression.1,2
The clinical evidence is convincing that our patients developed autoimmune hypophysitis as a consequence of anti-CTLA-4 antibody infusion. The cellular mechanisms of anti-CTLA-4 antibody that can generate tumor regression and autoimmunity have not been clearly elucidated. It is known that mice deficient in CTLA-4 develop autoimmunity and lethal lymphoproliferative disease.11–13 It has also been recognized that CTLA-4 gene polymorphisms in human beings can be associated with autoimmune diseases.14 Based on a phase 1 trial of anti-CTLA-4 antibody and vaccinations with multiple melanoma peptides in patients with stage III or resected stage IV melanoma, Sanderson et al15 have suggested that polymorphisms for CTLA-4 correlate with the development of autoimmunity in these patients. Another possibility for the development of hypophysitis in our patients may be autoimmune antibodies directed against the pituitary gland. Previous reports have shown that anti-CTLA-4 antibody can stimulate low titers of autoantibodies.16 It is our plan to determine the presence or absence of anti-pituitary antibodies in these patients.
Based on our experience, we recommend that patients receiving anti-CTLA-4 antibody be closely monitored for autoimmune endocrinopathies. Baseline and sequential levels of cortisol, corticotropin, free thyroxine, and TSH as well as testosterone should be obtained. Baseline MRI evaluation of the pituitary gland should be obtained, and repeat MRI evaluation should be considered in patients with evidence of panhypopituitarism or with complaints of headaches or visual changes. The MRI finding of pituitary enlargement may precede laboratory or clinical evidence of autoimmune hypophysitis.
The optimal treatment of autoimmune hypophysitis associated with CTLA-4 blockade remains to be defined. Patients receiving anti-CTLA-4 antibody who show evidence of autoimmune hypophysitis require that the antibody infusions be discontinued and that the necessary physiologic hormone replacements be instituted. We have been encouraged by the fact that 3 patients treated with a short course of high-dose dexamethasone followed by physiologic hormone replacement have demonstrated partial recovery of pituitary function, and this remains our current treatment strategy.
Our group has documented that infusion of human anti-CTLA-4 monoclonal antibody can mediate objective tumor responses in patients with advanced renal cancer and melanoma and that this therapy is associated with autoimmune hypophysitis as well as other autoimmune toxicities. Of the 8 patients reported here with autoimmune hypophysitis, 5 had objective tumor responses. This is consistent with previous reports that document an association between autoimmunity and tumor response.1,2 We are continuing to investigate the potential use of this antibody for the treatment of patients with cancer.
References
- 1.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-CTLA-4. J Clin Oncol. doi: 10.1200/JCO.2005.06.205. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goudie EB, Pinkerton PH. Anterior hypophysitis and Hashimoto's disease in a young woman. J Pathol Bacteriol. 1962;83:584–585. [PubMed] [Google Scholar]
- 4.Thoudou E, Asa S, Kontogeorgos G, et al. Clinical case seminar: lymphocytic hypophysitis: clinicopathological findings. J Clin Endocrinol Metab. 1995;80:2302–2311. doi: 10.1210/jcem.80.8.7629223. [DOI] [PubMed] [Google Scholar]
- 5.Abe T, Matsumoto K, Sanno N, et al. Lymphocytic hypophysitis: case report. Neurosurgery. 1995;36:1016–1019. doi: 10.1227/00006123-199505000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Ezzat S, Josse RG. Autoimmune hypophysitis. Trends Endocrinol Metab. 1997;8:74–80. doi: 10.1016/s1043-2760(96)00270-6. [DOI] [PubMed] [Google Scholar]
- 7.Schloot N, Eisenbarth GS. Isohormonal therapy of endocrine autoimmunity. Immunol Today. 1995;16:289–294. doi: 10.1016/0167-5699(95)80183-9. [DOI] [PubMed] [Google Scholar]
- 8.Kristof RA, Van Roost D, Klingmuller D, et al. Lymphocytic hypophysitis: non-invasive diagnosis and treatment by high dose methylprednisolone pulse therapy. J Neurol Neurosurg Psychiatry. 1999;67:398–402. doi: 10.1136/jnnp.67.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward L, Paquette J, Seidman E, et al. Severe autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy in an adolescent girl with a novel AIRE mutation: response to immunosuppressive therapy. J Clin Endocrinol Metab. 1999;84:844–852. doi: 10.1210/jcem.84.3.5580. [DOI] [PubMed] [Google Scholar]
- 10.Chan WB, Cockram CS. Panhypopituitarism in association with interferon-alpha treatment. Singapore Med J. 2004;45:93–94. [PubMed] [Google Scholar]
- 11.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 12.Chamber CA, Sullivan TJ, Allison JP. Lymphoproliferation in CTLA-4 deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity. 1997;7:885–895. doi: 10.1016/s1074-7613(00)80406-9. [DOI] [PubMed] [Google Scholar]
- 13.Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 14.Ueda H, Howson JM, Esposito L, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 15.Sanderson K, Scotland R, Lee P, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and montanide ISA 51 for patients with resected stage III and IV melanoma. J Clin Orthod. 2005;23:741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 16.Hodi FS, Mihm MC, Soiffer RJ, et al. Biological activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]


