Despite advances in elucidating the structural basis of ion permeation and pore block, the structural and mechanistic basis for regulating ion channel gating has remained a more elusive problem. For the voltage- dependent K+ channel superfamily, two major categories of signal, voltage and ligand, are known to regulate gating. Progress on the structural elements involved in gating regulation have been aided immensely by the fact that the key gating regulators, voltage sensors and ligand-binding domains, are largely modular components that are “simply” appended to the basic minimal two transmembrane (2TM) K+ channel pore motif. Nevertheless, one impediment to the structural understanding of channel gating mechanisms is that the bacterial channels most amenable to full structural determination have not proven as favorable for the required functional studies of the allosteric regulation of channel gating. An article from the laboratory of Youxing Jiang in the current issue of the Journal (see Li et al. on p. 109) suggests that, at least for the Ca2+-activated MthK channel, this situation is about to change.
The structure of MthK, a K+ channel from Methanobacterium thermoautotrophicum, was solved by Jiang and coworkers while in Rod MacKinnon's laboratory (Jiang et al., 2002a). The structure contains a C-terminal cytosolic ligand-binding domain homologous to eukaryotic NAD binding domains, but named RCK for their role in regulation of conductance for K+ (Jiang et al., 2001). This generated considerable excitement, as it was also suggested that eukaryotic channels of the Slo (or BK) channel family may contain a somewhat homologous cytosolic domain (Jiang et al., 2001, 2002a; Pico, 2003). This is important because, although biophysical studies of BK channels have considerably advanced our understanding of allosteric regulatory mechanisms (Horrigan and Aldrich, 2002), this occurred in the absence of real structural information. In contrast, MthK has provided a number of structural insights while being limited in regards to functional studies. Now with a set of three papers published over the past year, the Jiang laboratory has substantially extended our understanding not only of ligand-dependent changes in structure of RCK-containing modules (Dong et al., 2005; Ye et al., 2006), but now establishes, along with work from another lab (Zadek and Nimigean, 2006), that the MthK channel may be suitable for a robust analysis of allosteric regulation of gating (Li et al., 2007). For the RCK-regulated channels, this should allow the necessary correlation of functional studies of channel behavior with companion structural and biochemical studies.
RCK Domains: Regulator Domains of K+ Channels and Transporters
Why are RCK domains interesting? The underlying structural motif of the RCK domain is essentially identical to that of the classical nicotinamide dinucleotide binding motif observed in a large number of different prokaryotic and eukaryotic proteins, all containing an alternating βαβαβ Rossmann-fold structural arrangement (Bellamacina, 1996). Within bacteria, RCK-containing proteins involved in ion transport are predominantly of two types. In a number of adenine nucleotide–regulated transporter proteins, an RCK-containing domain is expressed as a separate cytosolic protein that regulates the transporter protein function. For proteins involved in regulation of coupled K+ transport, such cytosolic domains have also been termed KTN domains (K+ transport and NAD binding) (Bateman et al., 2000). However, RCK and KTN domains are essentially similar structures. An important example of an RCK-regulated K+ cotransport protein is the KtrAB cotransporter (Nakamura et al., 1998), in which the soluble KtrA protein is a separately expressible protein regulating transport function. Although KtrAB functions as a cotransporter, the KtrB transporter module exhibits a tetrameric linking of the classic 2TM K+ channel pore modules, and mutation of a glycine in a degenerate selectivity filter position results in inhibition of transport (Tholema et al., 2005). Thus, RCK-regulated transport proteins share features with their channel kin. In addition to their role in cotransport systems, RCK domains also contribute regulatory modules to a large fraction of all bacterial K+ channels (Kuo et al., 2005). For K+ channels, RCK domains have apparently been exploited to allow regulation by cytosolic ligands other than nucleotides and it is in this area that their relevance to channels may be most important.
Initial insights into the structural motifs of RCK domains in K+ channels occurred only about six years ago. The MacKinnon group focused on the large number of bacterial K+ channels that contained cytosolic domains with homology to the nucleotide binding domains (Jiang et al., 2001; Pico, 2003). The first success was obtained with the RCK domain from an Escherichia coli 6TM channel revealing that RCK domains assembled in homodimers with a characteristic clamshell-like coupling with the hinge between the shells resting on interlocked αF and αG helices (Jiang et al., 2001). This was complemented by work from another group on a truncated form of the KtrA cytosolic protein of the KtrAB transporter (Roosild et al., 2002), which revealed a very similar βα pattern with two KtrA subunits coupled in a dimeric hinge very similar to the homodimeric RCK domains. In the KtrA study, it was proposed that nucleotide binding resulted in a switch from a dimer to a tetramer, with the conformational change underlying transporter regulation. Subsequently, Jiang et al. (2002a) presented the open channel structure of the Ca2+-liganded MthK channel, also showing electrophysiologically that channel openings are activated by millimolar Ca2+. This showed directly that RCK domains can mediate regulation by ligands other than nucleotides and, specifically, by cytosolic cations. This work presented the landmark conclusion that the architecture of the RCK domain assembly was octameric, with the four domains (inner RCK domains) closest to the channel being directly linked to the inner helices of the 2TM pore modules, while another four domains (outer RCK domains) were acquired from the cytosol. This arises because of a secondary translation initiation site on the MthK gene downstream from the pore domain sequence that results in synthesis of RCK domains lacking the pore module. Addressing this issue, in one of the recent papers (Dong et al., 2005) Jiang and coworkers point out that, in addition to MthK, a number of other RCK-containing K+ channels contain secondary initiation sites. Their results also show that the physiological ionic conditions that promote octamerization also strongly favor channel activation.
Another important contribution of the earlier MthK work was a proposal regarding the conformational changes that may occur in the RCK octamer during MthK gating (Jiang et al., 2002a,b). To generate a model of a closed channel RCK octamer, the RCK homodimer from the E. coli 6TM K+ channel (Jiang et al., 2001) was used and then compared with the open MthK octameric structure. The basic MthK octamer exhibits an alternating assembly of inner and outer RCK domains arranged in a so-called gating ring with essentially two types of interfaces between RCK domains. Each RCK domain type (inner or outer) will interface with a pair of RCK domains of the other type. Based on differences in the closed channel model and the open MthK structure, the interfaces were observed to be of two types. One type was termed a flexible interface reflecting the homodimeric pair containing symmetric Ca2+ binding sites. The second type was initially termed a fixed interface (although now termed assembly interface, see below). Although this model has been provocative, clarification of the conformational changes occurring in the MthK octamer during gating has required structural information about MthK in closed conformations. This has been the direction taken by the Jiang laboratory, which has used a combination of x-ray structural studies, biochemical studies, and electrophysiological studies of channel activity in order to understand RCK domain–regulated channel gating.
New Views of the MthK Channel from the Jiang Lab
As a first step, the Jiang lab obtained three structures of isolated MthK RCK domains lacking the pore module (Dong et al., 2005). Two structures (R32 and P21 crystal forms) were obtained at low pH with 0 Ca2+; a third (also a P21 crystal form) was obtained at low pH and 20 mM Ca2+ (Table I). In each case, a characteristic homodimer was formed with the bilobed, clamshell-like coupling between monomers. The Ca2+-liganded and unliganded P21 homodimers were essentially identical with a somewhat more open dimer when compared with RCK domains across the flexible interface in the original Ca2+-liganded MthK structure (Jiang et al., 2002a). In contrast, the R32 crystal revealed a more closed clamshell, consistent with a closed channel conformation. These structures provide a higher resolution view of key details of the MthK homodimer, revealing more explicitly the structural details of the Ca2+ binding sites and the points of interaction at the flexible interface. The differences in the clamshell inside angle between different structures confirm that the homodimer interface is flexible. The structural results were also complemented with biochemical studies that established conditions under which a dimer to octamer transition could be demonstrated using size exclusion chromatography or circular dichroism. At pH 4.5, RCK domains assembled into homodimers, while at pH 8.0, RCK domains form octamers, suggesting that assembly into octamers may occur in the physiological pH range. In addition, Ca2+ in the range of 0.1 to 20 mM Ca2+, but not Mg2+ or Ba2+, also promotes a similar assembly into octamers. As shown in this issue (Li et al., 2007), this range of pH and [Ca2+] produces marked effects on MthK channel activation.
TABLE I.
The Library of MthK Snapshots
| Structure | Open octamer | R32 dimer | P21 dimer | P21 dimer | Closed octamer | Partially open octamer |
|---|---|---|---|---|---|---|
| Construct | Intact pore | No pore domain | No pore domain | No pore domain | D184N no pore | D184N no pore |
| Resolution | 3.3 Å | 2.8 Å | 1.7 Å | 2.1 Å | 2.8 Å | 2.8 Å |
| pH | 6.5 | 5.5 | 4.5 | 4.5 | 8.0 | 8.0 |
| Ca2+ | 200 mM | 0 Ca2+ | 20 mM | 0 Ca2+ | 0 Ca2+ | 0 Ca2+ |
| Dimer hinge inner angle | 88° | 70° | 102° | |||
| R116-R116 | 74 Å | 66 Å | ||||
| Presumed conformation | Open | Closed | Open | Open | Closed | Partially open |
| PDB ID and reference | 1LNQ (Jiang et al., 2002) |
2AEM (Dong et al., 2005) |
2AEF (Dong et al., 2005) |
2AEJ (Dong et al., 2005) |
2FY8 (Ye et al., 2006) |
2FY8 (Ye et al., 2006) |
The next advance was a crystal structure of a Ca2+-free octameric MthK gating ring (Ye et al., 2006). Intriguingly, two distinct conformations were detected in the crystals, one termed a closed gating ring and the second, a partially open gating ring. In the latter, a single dimer adopts an unusual structure in which the clamshell is even more wide open than in the open channel structure. These structures were obtained in the absence of Ca2+, but at pH 8.0, and also required a mutation of one of the Ca2+ binding site residues, D184N (Table I). The structures reveal several important new aspects of gating ring assembly. First, the results show that conformational changes do occur at the interdimer fixed interfaces. As a consequence, Ye et al. (2006) rename the fixed interfaces as “assembly” interfaces, consistent with the idea that they are involved in the assembly of homodimers into octamers. Second, in the closed structure, a new interface is observed between adjacent pairs of RCK domains around either the inner set of RCK domains or outer set. These interactions involve largely salt bridges and hydrogen bonds and must apparently be disrupted during opening of the gating ring. Finally, the overall consequence of the conformational changes between the closed and open conformation is that the distance between diagonal arginines that define the beginning of each inner RCK domain increases from 66 to 74 Å. This expansion fits with the gating ring model of MthK activation (Jiang et al., 2002a) in which conformational changes in the gating ring tug on linkers between the inner RCK domains and the pore-lining inner helices, leading to channel opening.
How certain can we be regarding the functional equivalent of the conformations revealed by the new crystal structures? Comparison of the Ca2+-free octamer and the previously published Ca2+-liganded octamer support the idea that conformational changes at the flexible dimer and elsewhere lead to a gating ring expansion. However, the presence or absence of Ca2+ is not the only determinant of MthK's conformational status; pH appears to have a marked effect, not only on octamer formation, but also on channel activity. The new Ca2+-free structure was obtained at pH 8.0, a condition that results in fairly robust MthK gating, and one wonders whether there may be a protonated, Ca2+-free octameric structure that differs from those so far obtained. Also what is the significance of the wide-open dimer, which is proposed to represent some gating intermediary between closed and open conformations? Perhaps the dimer can open to this extent because it is not tethered to the pore, as would be the case in a native channel. Despite these questions, the new structures only fuel excitement regarding the possibilities that the MthK channel offers for teasing apart structural correlates of gating intermediates.
Li et al. (2007) now examine a number of functional properties of single MthK channels, including rectification and gating. My focus here is on the gating behavior, because that reflects on the RCK domains. Previous work suggested that Ca2+ only weakly activated the MthK channel (Jiang et al., 2002a). The new work now provides direct measurements of the effect of pH and Ca2+ on MthK open probability. At pH 7.0, Ca2+ up to 10 mM activates the channel only weakly. At pH 8.0, Ca2+ is able to drive Po to near 1. Above 9.0, pH alone appears able to drive the channel to near maximum Po. Thus, conditions have been defined over which MthK gating can be examined over a wide range of open probability by two distinct signals, Ca2+ and pH. Importantly, Li et al. (2007) also isolated and expressed an MthK pore module in the absence of any RCK regulatory domain. In this case, infrequent channel openings of the appropriate conductance and rectification behavior are observed, and the opening frequency is not modulated by either Ca2+ or changes in pH. Thus, regulation by either pH or Ca2+ arises from the RCK domains. By appropriate manipulation of Ca2+ and pH, it should now be possible to more directly examine allosteric regulation of this channel.
One of the intriguing observations in this set of articles from Jiang' laboratory is that increases in channel activation are strongly correlated with conditions that influence biochemical conversion of the RCK domains from a largely dimeric organization to an octameric organization. One wonders whether the dimer–octamer transition might contribute to normal gating behavior. The dimer–octamer transition assays are done with isolated RCK domains, and it is possible that the dimer–octamer equilibrium may be strongly influenced by whether or not the inner RCK domains are tethered to a pore module. In terms of single channel gating behavior, both Ca2+ and increases in pH exert rather similar effects, but there are differences. In the absence of Ca2+, elevations in pH appear able to drive channel open probability to near maximal levels. In contrast, the maximal Po driven by increases in Ca2+ varies in a pH-dependent fashion. Li et al. are cautious in their interpretation of the separate effects of pH and Ca2+, but propose three general types of conformational changes. First, pH is proposed to regulate the assembly of the homodimers into a closed octameric structure. Second, both pH and Ca2+ are proposed to influence transitions from the closed form of the gating ring to the more wide open gating ring necessary for gating. Third, rapid gating events observed in single channel recordings, that are largely independent of either pH or Ca2+, are proposed to involve gating at the MthK selectivity filter.
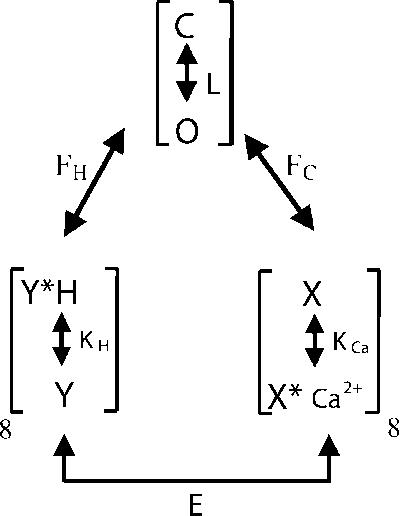
The ability of two distinct signals to influence MthK gating is reminiscent of the regulation observed for BK channels by voltage and Ca2+ (Horrigan and Aldrich, 2002) and for Slo3 channels by voltage and pH (Zhang et al., 2006). In both cases, two allosteric regulators independently regulate channel activation. Scheme 1 illustrates such an allosteric model recast to consider independent pH (Kd(H+)) and Ca2+ (Kd(Ca2+)) binding equilibria regulating a channel closed–open equilibrium (L), with allosteric coupling constants (FH, FC, and E) linking each equilibrium. Eq. 1 provides the equilibrium predictions for channel Po for such a model, assuming eight sensing elements for each ligand.
 |
(SCHEME 1) |
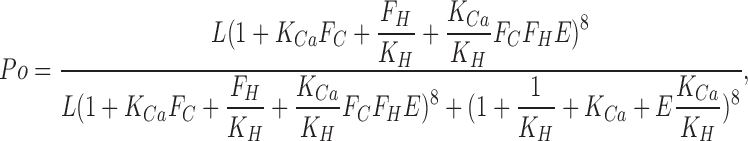 |
(1) |
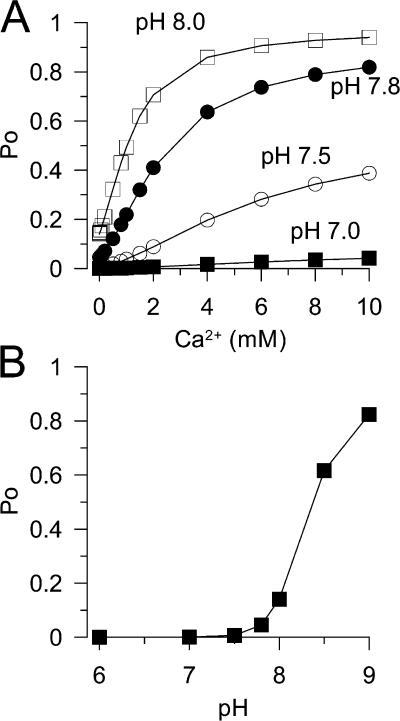
where KCa = [Ca2+]/Kd(Ca2+) and KH = [H+]/Kd(H+). With Ca2+ and H+ dissociation constants appropriate for MthK (Kd(Ca2+) = 3 mM; Kd(H+) = 0.01 μM), this simple scheme yields estimates of the Ca2+ and pH dependence (Fig. 1, A and B) of Po that can reproduce some key aspects of the MthK behavior. Specifically, the variation in the saturating Ca2+-dependent Po as a function of pH is reproduced, as well as the near maximal Po values by high pH in the absence of Ca2+. This supports the idea that each sensor independently regulates the channel closed–open equilibrium. However, there are important aspects of the MthK behavior that imply a more complex relationship between effects of pH and Ca2+. Specifically, at low pH, the Hill coefficient for activation by Ca2+ is very high, decreasing as pH is increased (Li et al., 2007). A steep Hill coefficient at low pH has also been noted in recent work from the Nimigean laboratory (Zadek and Nimigean, 2006). It will be important to resolve the structural and allosteric basis for these unique aspects of the regulation of the MthK channel by pH and Ca2+.
Figure 1.
A simple allosteric model involving independent regulation by pH and Ca2+ approximates some, but not all, features of MthK steady-state Po. In A, the predicted Po as a function of Ca2+ is shown for four different pHs (7.0, 7.5, 7.8, and 8.). Coupling constants were E = 1, FH = 4.5, FC = 2, KCa =3 mM, KH = 0.01 (pH 8.0); L = 5 * 10−5. The model successfully predicts different Ca2+-dependent limiting Po for different pH. However, the Hill coefficients for Po vs. Ca2+ curves become smaller at higher pH, in contrast to the MthK data (Li et al., 2007). In B, the predicted Po as a function of pH, in the absence of Ca2+, is displayed, closely approximating the observed data.
Lessons from the KtrAB RCK-containing K+ Transport Protein
The implications of this new information about MthK take on added significance in light of recent work on the KtrA RCK domain, where new structures of the KtrA protein have been determined, again revealing an octameric arrangement of a set of four symmetrical homodimers (Albright et al., 2006). The basic octameric arrangement shares marked similarities with MthK, involving alternating interfaces, similar to the flexible and assembly interfaces. Furthermore, like for MthK, assembly of the KtrA dimers into an octamer is driven by increases in pH. For KtrA, three forms of octameric ring structures, a square form (ATP bound), a diamond form (NADH bound), and a rectangular form (also with NADH), were obtained. Superposition of individual monomers within the rings shows almost no differences, but, like MthK, the change in octamer structure is associated with changes at the flexible dimer hinge. Given that each crystal form contains bound nucleotide, it is more difficult to assign functional significance to particular conformations for KtrA. However, the differences in the KtrA flexible dimer interfaces that occur between the rectangular form and the square form (the diamond form is intermediate) are consistent with the MthK transition from the unliganded, closed form to the Ca2+-bound, open form. On balance, the work indicates that, as suggested by the Jiang group, the octameric arrangement of RCK domains may be the critical architectural arrangement of RCK domains that allows them to mediate regulation of protein function. Central to both the MthK and KtrA observations is the idea that ligand binding within the clamshell of a homodimer pair results in a conformational change in the flexible interface that is coupled to overall conformational changes in an octameric gating ring.
Three important differences between the KtrA octamer and that of MthK need to be mentioned. First, the KtrA protein for which a structure has been generated is a truncated protein lacking the C-terminal lobe of the basic RCK motif. Thus, the extra C-terminal lobe structure resting below the clamshell hinge of the MthK dimer is absent. Although the absence of the C-terminal lobe could result in a somewhat unnatural structure, the similarity of the KtrA truncated dimer to the MthK dimer suggests that the absence of the C-terminal lobe of an RCK domain does not alter the essential nature of the N-terminal lobe clamshell. Second, in contrast to the direct linkage between the MthK RCK domains and the pore module, direct covalent mechanical linkages between the KtrA RCK domain and the KtrB transport module do not occur and are not required to regulate transport. Third, based on biochemical experiments defining the stoichiometry of the KtrAB protein, it appears that two KtrB transport modules are associated with each KtrA octamer.
Central to both the MthK and the KtrA results is that ligand binding results in a conformational change in the gating ring that is driven by the opening of the dimer clamshell. For MthK, this movement increases the opening distance between an arginine residue (R116; Table I) that defines the beginning of the RCK domains and connects the RCK domains to the pore domain inner helix. For KtrA, it remains unclear how this movement might regulate transport activity, particularly given the possibility that a single KtrA octamer may regulate transport in a pair of KtrB transporters. For many cases in which cytosolic domains have ligand-dependent regulatory effects on K+ channels, the regulatory domains are tethered to the channel via a linker connected to the inner pore domain helix. This has naturally led to the idea that regulatory effects on gating mediated by conformational changes in the cytosolic domain are coupled to the pore domain by changes in tension on the inner helix mediated by tugging on the linker. Evidence in support of this notion has been obtained for the BK channel through experiments supporting the view that shortening the linker length favors channel activation, while lengthening the linker length reduces channel activation (Niu et al., 2004). A lesson from the KtrAB protein may be that RCK-containing octamers can regulate channel-like transporter function without direct mechanical linkages to inner helices.
Together, the results on MthK and KtrAB raise some important considerations. Among these is whether the octameric assembly of RCK domains may be fundamental to the function of all RCK-containing transport proteins. Structural information on other RCK-containing proteins is required to test this idea. In addition, for those studying channels, it may be useful to keep in mind that although the physical linkage between RCK domains and the inner helix in K+ channels provides a satisfying conception for how conformational changes in the gating ring may be coupled to channel regulation, the KtrA results show that other sorts of important functional interactions between an RCK octamer and a pore module can occur.
Relevance to Slo Family Channels
Is any of this relevant to the Slo family of eukaryotic channels of which the BK channel is the best known example? Long ago it was shown that the C terminus of each BK α subunit contains two modular components that can be expressed as distinct proteins, while retaining normal channel function (Wei et al., 1994). Two large segments within the C terminus of a BK α subunit are highly conserved across BK channel genes among different species from Caenorhabditis elegans to humans, while intervening sequence connecting the two domains shows substantial length variation and little homology. It now appears that these components reflect the possible presence of two RCK-like domains within the two segments of each BK α subunit. During examination of the RCK domain of the E. coli 6TM K+ channel (Jiang et al., 2001), it was proposed that at least one and likely two RCK domains may be present within the BK C terminus. Subsequently, acidic residues in the first RCK domain were shown to influence Ca2+ sensitivity of the BK channel (Xia et al., 2002), suggesting that this first RCK domain (RCK1) may contain a ligand binding site, while a second putative Ca2+ sensing site, termed the Ca2+ bowl (Schreiber and Salkoff, 1997; Xia et al., 2002; Bao et al., 2004), had previously been described at a more distal position on the C terminus. The position of the second RCK domain within the BK C terminus and its potential relationship to the Ca2+ bowl has been described more recently, although there are some important discrepancies in details of the exact alignments (Pico, 2003; Roosild et al., 2004; Kim et al., 2006). Within the putative BK RCK2 domain, the various alignments generally agree up through the αD helix. However, in one alignment (Pico, 2003), additional insertions in the putative RCK2 domain result in the Ca2+ bowl segment following the βE sheet, whereas in another alignment (Kim et al., 2006) the Ca2+ bowl is positioned following the αG helix. This results in distinctly different predictions as to where the presumed Ca2+ bowl–sensing residues would be positioned within the structure of any flexible dimer. Irrespective of this discrepancy, the idea that each BK α subunit contains two RCK-like domains, resulting in an octameric arrangement of domains, similar to those observed for MthK and KtrA, provides a useful guide for thinking about BK channel ligand dependence. Yet, a more cautious view is that homology between proposed BK channel RCK domains and other RCK proteins is insufficient to justify assertion of an RCK-type structure in BK channels (Fodor and Aldrich, 2006).
If we accept the gating ring model for BK channels, what might the insights from the MthK and KtrA gating rings suggest about the organization of RCK domains in BK channels? The BK RCK1 domains would correspond to the inner RCK domains of the bacterial octameric gating rings, whereas the putative RCK2 domains would correspond to outer domains. Each RCK1 domain presumably is coupled to one RCK2 domain to form a dimer corresponding to the flexible interface and then another RCK2 domain to form an assembly interface. An important question articulated in a recent paper from the Magleby lab (Qian et al., 2006) concerns whether the dimer pairs corresponding to the flexible interface arise from an RCK1 and RCK2 domain on a single α subunit, or RCK1 and RCK2 domains on adjacent subunits. To address this question, Qian et al. (2006) examined the Ca2+ dependence of activation of single BK channels with two active RCK1 Ca2+ sensors and two active Ca2+ bowl sensors (associated with the putative RCK2 domains). Such channels were engineered to be of two types: ones in which both the RCK1 and RCK2 sensors were on the same α subunit and ones where the active sensors were on different subunits. When both Ca2+ sensors are on the same subunit, a modest cooperativity in the Ca2+ dependence of activation is observed. Assuming that Ca2+ binds on both sides of the flexible interface, as in MthK, an analysis of the possible arrangements of the functional and mutated high affinity Ca binding sites on the potential RCK1 and RCK2 interfaces together with the physiological response was consistent with the flexible interface forming between RCK domains on the same subunit. The length and flexibility of the linker between the two modules of the C terminus (Wei et al., 1994) would presumably permit the RCK1 and RCK2 domains within a single subunit to form the appropriate ligand-regulated flexible interface. However, for an RCK1/RCK2 flexible hinge to occur in BK channels, whether from the same or different subunits, would require two novel features: first, the dimer would exhibit substantial asymmetry and, second, the two presumed Ca2+-sensing sites would not be positioned symmetrically above the flexible hinge. The lack of symmetry would be true irrespective of the position of the Ca2+ bowl within the alignment of the RCK2 domain (Pico, 2003; Kim et al., 2006).
To test the applicability of the gating ring hypothesis to BK channels, Kim et al. (2006) (also see Pico, 2003) employed a mutant cycle analysis to test potential interactions between helices in the C terminus. Residues proposed to mediate the fixed (now assembly) interfaces in RCK1 and RCK2 domains were shown to interact in an energetically nonadditive fashion, supporting the idea that these two parts of the BK cytosolic domain do interact. Although such results suggest that the architecture of the BK cytosolic domains may mirror the general principles of the bacterial octameric RCK gating rings, the harsh reality remains that this postulated relationship is just a conjecture. Perhaps the new information from bacterial RCK domains may provide insight into strategies of defining reduced components of the BK cytosolic domains that may be suitable for structural determinations.
The structural underpinnings for the allosteric gating behavior of any ion channel are only just beginning to emerge and how conformational changes in different parts of ion channel are coupled remains speculative. As noted by Jiang and colleagues, crystal structures represent only snapshots of possible conformational states a protein may traverse during normal function. Yet, an exciting aspect of this most recent study is that it may now be possible to correlate the gating behavior of the MthK channel with specific conformational states observed through crystallization. This work points to a fruitful coupling of structure, biochemistry, and biophysics to illuminate how regulation of a channel by an RCK-containing cytosolic structure occurs. Single structures are not enough and care must be taken regarding conclusions about what particular snapshots of structure actually reflect.
For regulatory modules, there are two fundamental questions that must be addressed. First, what structures does the module adopt during normal gating behavior? Second, how do those structural changes produce the opening or closing of the channel? Addressing those questions requires intimate knowledge not only of structure, but also the biophysical properties of allosteric regulation of the channel. The new MthK structures clearly define a number of points of interaction in the dimers and octamers that will certainly be points of attack in future attempts to simultaneously manipulate structure, biochemistry, and function. Overall, the studies from Jiang's lab represent an impressive and systematic approach toward unlocking the nuances of RCK-mediated allosteric regulation of K+ channels.
Abbreviations used in this paper: KTN, K+ transport and NAD binding; RCK, regulation of conductance for K+; TM, transmembrane.
References
- Albright, R.A., J.L. Ibar, C.U. Kim, S.M. Gruner, and J.H. Morais-Cabral. 2006. The RCK domain of the KtrAB K+ transporter: multiple conformations of an octameric ring. Cell. 126:1147–1159. [DOI] [PubMed] [Google Scholar]
- Bao, L., C. Kaldany, E.C. Holmstrand, and D.H. Cox. 2004. Mapping the BKCa channel's “Ca2+ bowl”: side-chains essential for Ca2+ sensing. J. Gen. Physiol. 123:475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman, A., E. Birney, R. Durbin, S.R. Eddy, K.L. Howe, and E.L. Sonnhammer. 2000. The Pfam protein families database. Nucleic Acids Res. 28:263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamacina, C.R. 1996. The nicotinamide dinucleotide binding motif: a comparison of nucleotide binding proteins. FASEB J. 10:1257–1269. [DOI] [PubMed] [Google Scholar]
- Dong, J., N. Shi, I. Berke, L. Chen, and Y. Jiang. 2005. Structures of the MthK RCK domain and the effect of Ca2+ on gating ring stability. J. Biol. Chem. 280:41716–41724. [DOI] [PubMed] [Google Scholar]
- Fodor, A.A., and R.W. Aldrich. 2006. Statistical limits to the identification of ion channel domains by sequence similarity. J. Gen. Physiol. 127:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan, F., and R. Aldrich. 2002. Coupling between voltage-sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J. Gen. Physiol. 120:267–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, M. Cadene, B.T. Chait, and R. MacKinnon. 2002. a. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 417:515–522. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, M. Cadene, B.T. Chait, and R. MacKinnon. 2002. b. The open pore conformation of potassium channels. Nature. 417:523–526. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., A. Pico, M. Cadene, B.T. Chait, and R. MacKinnon. 2001. Structure of the RCK domain from the E. coli K+ channel and demonstration of its presence in the human BK channel. Neuron. 29:593–601. [DOI] [PubMed] [Google Scholar]
- Kim, H.J., H.H. Lim, S.H. Rho, S.H. Eom, and C.S. Park. 2006. Hydrophobic interface between two regulators of K+ conductance domains critical for calcium-dependent activation of large conductance Ca2+-activated K+ channels. J. Biol. Chem. 281:38573–38581. [DOI] [PubMed] [Google Scholar]
- Kuo, M.M., W.J. Haynes, S.H. Loukin, C. Kung, and Y. Saimi. 2005. Prokaryotic K(+) channels: from crystal structures to diversity. FEMS Microbiol. Rev. 29:961–985. [DOI] [PubMed] [Google Scholar]
- Li, Y., I. Berke, L. Chen, and Y. Jiang. 2007. Gating and inward rectifying properties of the MthK K+ channel with and without the gating ring. J. Gen. Physiol. 129:109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, T., R. Yuda, T. Unemoto, and E.P. Bakker. 1998. KtrAB, a new type of bacterial K+-uptake system from Vibrio alginolyticus. J. Bacteriol. 180:3491–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, X., X. Qian, and K.L. Magleby. 2004. Linker-gating ring complex as passive spring and Ca2+-dependent machine for a voltage- and Ca2+-activated potassium channel. Neuron. 42:745–756. [DOI] [PubMed] [Google Scholar]
- Pico, A. 2003. RCK domain model of calcium activation in BK channels. Ph.D. thesis. The Rockefeller University, New York. 106 pp.
- Qian, X., X. Niu, and K.L. Magleby. 2006. Intra- and intersubunit cooperativity in activation of BK channels by Ca2+. J. Gen. Physiol. 128:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosild, T.P., K.T. Le, and S. Choe. 2004. Cytoplasmic gatekeepers of K+-channel flux: a structural perspective. Trends Biochem. Sci. 29:39–45. [DOI] [PubMed] [Google Scholar]
- Roosild, T.P., S. Miller, I.R. Booth, and S. Choe. 2002. A mechanism of regulating transmembrane potassium flux through a ligand-mediated conformational switch. Cell. 109:781–791. [DOI] [PubMed] [Google Scholar]
- Schreiber, M., and L. Salkoff. 1997. A novel calcium-sensing domain in the BK channel. Biophys. J. 73:1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholema, N., M. Vor der Bruggen, P. Maser, T. Nakamura, J.I. Schroeder, H. Kobayashi, N. Uozumi, and E.P. Bakker. 2005. All four putative selectivity filter glycine residues in KtrB are essential for high affinity and selective K+ uptake by the KtrAB system from Vibrio alginolyticus. J. Biol. Chem. 280:41146–41154. [DOI] [PubMed] [Google Scholar]
- Wei, A., C. Solaro, C. Lingle, and L. Salkoff. 1994. Calcium sensitivity of BK-type KCa channels determined by a separable domain. Neuron. 13:671–681. [DOI] [PubMed] [Google Scholar]
- Xia, X.-M., X.-H. Zeng, and C.J. Lingle. 2002. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 418:880–884. [DOI] [PubMed] [Google Scholar]
- Ye, S., Y. Li, L. Chen, and Y. Jiang. 2006. Crystal structures of a ligand-free MthK gating ring: insights into the ligand gating mechanism of K+ channels. Cell. 126:1161–1173. [DOI] [PubMed] [Google Scholar]
- Zadek, B., and C.M. Nimigean. 2006. Calcium-dependent gating of MthK, a prokaryotic potassium channel. J. Gen. Physiol. 127:673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., X.-H. Zeng, and C.J. Lingle. 2006. Slo3 K+ channels: voltage and pH dependence of macroscopic currents. J. Gen. Physiol. 128:317–336. [DOI] [PMC free article] [PubMed] [Google Scholar]