Abstract
Explanations for the evolution of human pygmies continue to be a matter of controversy, recently fuelled by the disagreements surrounding the interpretation of the fossil hominin Homo floresiensis. Traditional hypotheses assume that the small body size of human pygmies is an adaptation to special challenges, such as thermoregulation, locomotion in dense forests, or endurance against starvation. Here, we present an analysis of stature, growth, and individual fitness for a large population of Aeta and a smaller one of Batak from the Philippines and compare it with data on other pygmy groups accumulated by anthropologists for a century. The results challenge traditional explanations of human pygmy body size. We argue that human pygmy populations and adaptations evolved independently as the result of a life history tradeoff between the fertility benefits of larger body size against the costs of late growth cessation, under circumstances of significant young and adult mortality. Human pygmies do not appear to have evolved through positive selection for small stature—this was a by-product of selection for early onset of reproduction.
Keywords: body size, demography, mortality, height, fertility
Human pygmies are defined as populations having an average male height <155 cm (1, 2). The word pygmy is frequently used to describe African groups that live in the African tropical forests and subsist (or have until recently subsisted) on hunting and gathering, the best known being the Aka (Western pygmies) and Efe and Mbuti (Eastern pygmies) (1). There are, however, many populations exhibiting pygmy stature outside Africa, including ones in the Andaman Islands, Malaysia, Thailand, Indonesia, the Philippines, Papua New Guinea, Brazil, and Bolivia [ref. 3 and supporting information (SI) Fig. 4]. The small body size of human pygmies has been interpreted as an adaptation in itself, whether to living in dense tropical forests (4), thermoregulation (1), or endurance against starvation in low productivity environments (5). However, as described by Diamond (5), none of these explanations account for the worldwide distribution of human pygmies—some pygmy-sized populations are found outside forests, and many live in cool and dry areas; furthermore, long-standing poor nutrition does not necessarily lead to pygmy size, as shown by groups who, like certain pygmies, experience frequent food shortages (6–8) and yet are among the tallest populations in the world. The adaptive value of pygmy body size remains an unanswered question in human evolutionary biology, and one thrown into sharp relief by the recent discovery of a small-bodied fossil hominin population of Flores (9–11). This article presents the results of the first population-wide study of the growth, fertility and mortality of two pygmy groups, the Aeta and Batak from the Philippines (3), giving unique insights into the life history of these populations and the fitness associated with variation in their phenotype. The data from these two groups were compared with those available for African pygmies to explore three questions—whether short stature is adaptive among pygmies, whether pygmy growth rates and final stature reflect nutritional stress, and whether age of onset of reproduction and cessation of growth correlate with individual fitness.
Results
Pygmy Growth Curves: A Comparative Approach.
In most human populations, a large portion of variance in stature can be accounted for by nutritional status (12). Nutritional studies worldwide have shown the predictability of the growth response to stress—societies with very high caloric budgets show significant secular trends toward earlier growth cessation, faster body growth, and increased size, whereas populations under nutritional stress normally reduce growth rates, delay growth cessation, and show smaller adult body sizes (12). To test whether the growth pattern of pygmies is characteristic of nutritionally impaired populations, growth curves were constructed for the Aeta and compared with those for the Biaka (13) and Agta (14) pygmies and compared with the lower percentiles of the U.S. growth distribution, representing undernourished individuals who grow only to average adult pygmy size (womenHEIGHT = 140 cm, corresponding to the 0.01th percentile of the U.S. distribution) (15). The curves show that pygmies terminate growth at an earlier age than the U.S. under-nourished sample (Fig. 1). Although the National Center for Health Statistics 0.01th percentile achieves adult size at age 15, pygmy women grow at higher rates and achieve a similar adult height at 12–13 years of age. In fact, ages at growth cessation among the Aeta, Agta, and Biaka are similar to those observed in the extremely well nourished 95th percentile of the U.S. distribution, whose adult size exceeds pygmy size by >30 cm. Very similar results are observed in men (data not shown). Further evidence that pygmy size is not just a plastic response to nutritional stress is provided by a comparison with eastern African Pastoralists (16), such as the Turkana and the Maasai from Kenya, who rank amongst the tallest traditional human populations (data for women: Turkana = 166 cm, Maasai = 160 cm; worldwide average male body size of 2,503 ethnographic populations is 163.9 cm, average female body size of 434 ethnographic populations is 159.9 cm) [Human Diversity Project, unpublished data (Leverhulme Centre for Human Evolutionary Studies, Cambridge, U.K.) and SI Fig. 5]. Both the Aeta, Biaka, and eastern African Pastoralists rely on limited caloric budgets (6–8) and therefore grow at slower rates than average western European and U.S. populations (Fig. 1). Despite dramatic differences in adult body sizes (between a 25- and an 35-cm difference), female pygmies and eastern African Pastoralists show similar growth deficits from early infancy to age 12 compared with western European and U.S. populations. Although the Aeta, Agta, and Biaka growth curves are placed between the percentiles 0.1 and 1, eastern African Pastoralists compare to the 5th to 10th percentile of the U.S. distribution. However, from age 12 onwards, there is a clear divergence in the growth trajectories of the three groups. Eastern African Pastoralists continue to grow for 3–4 years after the Aeta, Agta, and Biaka growth curves level off, moving continuously up from the 7th to the 50th percentile of the U.S. distribution between ages 12 and 22, thereby achieving the average height of well nourished American adults. In contrast, pygmies do not extend their growth trajectories and stop growing as early as the U.S. 50th percentile (Fig. 1), dropping to the percentile 0.01 at adulthood. Thus, the smaller adult size of pygmies relative to eastern African Pastoralists is mostly caused by a difference in duration rather than rate of growth, the opposite of what is observed in cases of nutritionally induced stunting (12).
Fig. 1.
Growth curves for different human populations. Pygmy women [Aeta (black triangles), n = 214; Biaka (13) (gray triangles), n = 157; and Agta (14) (open triangles), n = 83] grow at rates bellow the 5th U.S. percentile (intermediate solid line), and their growth curves level off early at ≈13 years old, reaching an adult body size equivalent to the percentile 0.01 (lower solid line). African Pastoralists (16) [Massai (open squares) and Turkana (black squares), n = 228], also grow at slow rates compared with U.S. subjects but extend their growth trajectories and achieve final height equivalent to the U.S. 50th percentile (solid upper line).
Pygmy Mortality and Fertility Schedules.
Given these results, we investigated how early growth cessation correlates with other aspects of life history among pygmies. The second and perhaps most distinctive feature of known pygmy populations is their very high adult and preadult mortality rates, more comparable with values observed in chimpanzees (17) than in other human traditional populations (18–20) (Fig. 2a). Life expectancy at birth in human pygmy groups is very low [24.2, 16.5, 24.3, 16.6, 15.6, and 16 years in Batak (3), Aeta (3), Agta (21), Aka (1), Mbuti (22), and Efe (3), respectively, significantly different from eastern African Pastoralists and other non-pygmy hunter-gatherer populations (34.6, 37.1, and 47.5 years in !Kung (19), Ache (18), and Turkana (20), respectively]. Chances of surviving to adulthood are also markedly reduced in pygmies: whereas only 51% of Batak (3), 33% of Aeta (3), 47% of Agta (21), 40% of Aka (1) and Mbuti (22), and 30% of Efe (3) children are expected to survive to 15 years of age, in other foragers and eastern African Pastoralists, those values are 76% (Turkana) (20), 59% (Kung!) (19), and 64% (Ache) (18). Patterns of both prereproductive and adult mortality separate human pygmies from these other groups: life expectancy at adulthood (defined here as age 15 years) is, respectively, 29.5, 27.3, 22.5, 20, and 32.5 years in Batak (3), Aeta (3), Aka (1), Mbuti (22), and Efe (3), against 41.5, 45.8, and 46.6 years in !Kung (19), Ache (18), and Turkana (20), respectively. Shortened lifespans also reduce the number of pygmy women who reach the end of the reproductive period. Age at last reproduction in the Aeta averages 37.4 years (±7.3, n = 11), but only 13–31% of women in pygmy populations survive from birth to this age. However, Turkana women reproduce until as late as 40.3 years (±7, n = 60) (21), and 63% of women survive to that age. We argue that the precocity of death in pygmies is the key to the evolution of both pygmy size and life history. According to life history theory (23), age at first reproduction is set by natural selection as the result of two opposite forces. On one hand, extended growth and the resulting larger adult size engender fertility gains and reduced offspring mortality, implying a pressure for delayed reproductive onset. On the other hand, early age at first reproduction is advantageous for minimizing the likelihood of death before reproduction, and reducing generation time. Thus, small body size in pygmies can be explained as a life history tradeoff between extended growth and early reproduction, with the balance pending toward the latter because of their exceptionally high mortality rates. This hypothesis is supported by the third common feature of human pygmy populations: Age-specific fertility curves (3, 18, 20, 21) are shifted toward earlier ages, showing an earlier peak of fertility when compared with non-pygmy groups (Fig. 2b).
Fig. 2.
Age-specific survivorship (a) and age-specific fertility (b) for different human pygmies. Pygmy populations: Eastern African Pygmies (3) (gray triangles, dashed line), Western African Pygmies (1) (gray triangles, solid lines), Aeta (3) (black triangles, solid line), Batak (3) (black circles, solid line), and Agta (21) (open triangles, solid lines). Non-pygmy populations: !Kung (19) (gray square, solid line), Ache (18) (cross, dashed line), Turkana (20) (black square, dashed line), and chimpanzees (open circles, solid lines).
Modeling Fitness as a Function of Growth, Fertility, and Mortality Schedules in Human Pygmies.
We have presented empirical evidence supporting the hypothesis that pygmy body size results from a tradeoff between time invested in growth and in reproduction. However, because the legitimacy of adaptive hypotheses can be more properly tested through the measurement of fitness associated with different phenotypes, we used a mathematical model to investigate whether optimal or average age at first reproduction and adult body size can be predicted from the mortality schedule in one pygmy population (the Aeta from the Philippines, for which age-specific survivorship, fertility, and growth curves were constructed). The model estimates the effect of age at onset of reproduction on individual fitness of pygmy women by calculating the parameter r (fitness corrected by the intrinsic rate of population growth) from the Euler–Lotka equation (24), which includes age-specific survivorship (lx) and age-specific fertility (mx) as independent variables. We used a binary logistic regression to estimate the effect on fertility (mx) of age, age squared, and stature (25) instead of body weight (18). In this analysis, we assumed that observations from the same woman at different ages were statistically independent. We found a positive association between size and fertility in the Aeta and the Efe (linear regression of offspring number on female adult height, controlling by age; Aeta: r = 0.242, F = 9.34, P < 0.005, n = 154; Efe: r = 0.195, F = 4.34, P < 0.05, n = 110). No significant relationship was found between male fertility and height.
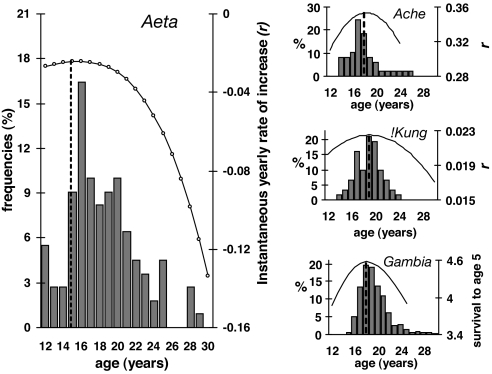
The model closely matches the actual distribution of age at first reproduction in the Aeta (Fig. 3). Female fitness peaks with an age at first reproduction of 15 years, much earlier than predicted for three other populations in which individual fitness was modeled as a function of age and body size, the Ache (18 years) (18), the !Kung (19.5 years) (19), and rural women in the Gambia (18 years) (25). The modal age at first reproduction in the Aeta pygmies is 16 years (n = 110), compared with 17 in the Ache, 19 in the !Kung, and 18 in the Gambian rural women. Our model also predicts higher fitness from very early ages at first reproduction (12 years, corresponding to the earliest recorded births in the population), as reflected in the real distribution of reproductive onset (Fig. 3). The precocity of reproduction in the pygmies is more evident when they are compared with the tall and slow-growing Turkana women, who start reproducing >4 years later than the Aeta (Turkana mean = 22.2 ± 3.3 years, n = 60 (20); Aeta mean = 18.5 ± 3.3 years, n = 110). Higher fertility at younger ages and increased fitness associated with an early age at first birth (16 years old) are only predicted for the well nourished, fast growing, and tall Americans (18) if they had remained under natural fertility conditions. The model demonstrates a selective advantage associated with earlier ages at first reproduction, which exceeds the fertility cost of the smaller adult size in the Aeta. In other words, although larger women are more fertile even among pygmies, the age-specific product of fertility and survivorship is greater in smaller Aeta women.
Fig. 3.
Fitness as a function of size achieved at first reproduction. (Left) estimated relative fitness (r) as a function of age at first birth for Aeta (solid line). The histogram shows observed frequencies of first birth in Aeta women; the dashed vertical line shows the predicted fitness peak (age at first birth = 15 years). (Right) Models (adapted from refs. 18 and 25) of relative fitness as a function of age and size at first birth for Ache, !Kung (18), and Gambian rural women (25).
Discussion
Based on their high mortality rates, relatively earlier growth cessation, and early peak of fertility, we postulate that pygmies occupy the “fast” extreme of life history strategies (26) among humans, with both longevity and resource availability as limiting factors. The resulting short stature is only one of the aspects of the fast pygmy life history package. The taller eastern African Pastoralists are an example of the opposite “slow” extreme, equally limited from the nutritional side but less constrained in terms of adult mortality. Whereas mortality rates were shown to affect rates of growth relative to adult body size in small-scale societies (27), here we propose that the small body size of human pygmies evolved as a by-product of selection for early onset of reproduction.
If our hypothesis is correct, the causes of the extremely high mortality rates among human pygmies need to be explained. It is here that the traditional hypotheses explaining the small body size of pygmies may prove useful. Although the challenges posed by thermoregulation, locomotion in dense forests, exposure to tropical diseases, and poor nutrition do not account for the characteristics of all pygmy populations, as pointed out by Diamond (5), they may jointly or partially contribute to the similarly high mortality rates in unrelated pygmy populations. We argue that the small body size of African and Asian pygmy populations evolved independently as a case of evolutionary convergence, resulting from a life history tradeoff between the fertility benefits of larger body size and the costs of late growth cessation under the circumstance of significant young and adult mortality.
Finally, the data presented here show that pygmy body size evolved through earlier cessation of growth, being therefore the result of changes in late rather than early stages of growth. This explains why brain growth, which is completed years before the onset of adolescence (28), is not affected in human pygmies (29). Therefore, if Homo floresiensis is a dwarfed form of Homo erectus, as proposed in ref. 29, the evolution of small body size on Flores could be understood as the life history consequence of ecological conditions in islands, such as increased extrinsic mortality rate and reduced resource availability (30); however, its small brain size and low encephalisation require the postulation of different adaptive mechanisms affecting earlier stages of development.
Materials and Methods
Data Collection.
The new data on the Aeta and Batak Negritos presented in the article were obtained during two seasons of fieldwork of four and five months in 2002 and 2003, respectively. Aeta and Batak individuals were measured to record their variation in body size and body proportions, and extensive interviews were conducted to record the genealogies and reproductive history of all adults.
Batak.
The Batak demographic data collected in this study was added to the data collected by J. Eder (Arizona State University, Tempe, AZ) in 1980, who kindly made his unpublished data available for this study. We visited three of five Batak villages and found 157 Batak. The number of Batak found by Eder in 1980 was 258, of whom we identified 129 individuals (including living and deceased people); this number corresponds exactly to 50% of the number of Batak described by Eder. The other 28 Batak were not in Eder's census because they were younger than 23 years old. Of the population of 129 individuals from Eder's census who were identified (directly interviewed or referred to in the genealogies), 41% (53) had died in the 23 year-interval between 1980 and 2003.
Aeta.
We visited 13 of 71 Aeta villages in Zambales; in these, we met 668 Aeta (including children, men, and women of all ages) [see SI Figs. 6 and 7 and SI Methods].
Anthropometry.
Growth curves.
A total of 199 Aeta girls and 146 Aeta boys from 0 to 20 years of age were measured by A.B.M. The Batak were not included in growth analyses because of the small number of children existent today. The data for other pygmy and non-pygmy groups are available in refs. 13, 14, and 16.
Adult body sizes.
A total of 209 Aeta women and 146 Aeta men older than 21 years of age had their height measured by using a movable Harpenden Anthropometer. Adult height was not included in any analyses for Batak population because of the small sample size.
Data on Individual Ages.
Aeta and Batak.
Ages were estimated as described in SI Table 1 and SI Methods.
Agta.
Exact ages are known for this population (14, 21). The growth, fertility, and mortality similarities among Agta, Aeta, and Batak show that the age estimates for both Aeta and Batak were precise enough to produce reliable analyses.
Other populations.
The age estimates for individuals of all other (Efe, Mbuti, etc.) pygmy groups included in the analyses were given by the researchers who carried out the original studies.
Life Tables.
Aeta and Batak.
Mortality and survivorship rates were estimated from death records only (19) for the Aeta (n = 239) and the Batak (n = 202). Very similar estimates were obtained by fitting data of living individuals to Weiss model life tables (23).
Efe.
From 1928 to 1938, the Efe from the Ituri forest (Eastern African Pygmies) were studied by Schebesta and colleagues (31, 32). From his data, we estimated average family size (6.6), population average age (26.6 years), adult average age (34.7 years), and proportion of the population >50 years of age (16.1%); these data were then fit to Weiss model life table MT:32.5–30 (22).
Mbuti.
The Mbuti were studied by Turnbull (4). Based on his data, Weiss (22) calculated average adult age, probability of dying in the first year of life, and percentage population <15 years of age to fit the Mbuti data to the model life table MT:20–40.
Aka.
Cavalli-Sforza (1) estimated average age among the Aka in 21 years and used this value to identify the Weiss model life table MT: 22.5–40.
The data for other pygmy and non-pygmy groups are available refs. 18–21.
Age-Specific Fertility.
Age-specific fertilities were calculated for both the Aeta (n = 191 women) and the Batak (n = 30 women) divided in 5-year intervals, following the methodology described in Hill and Hurtado (18). The data for other pygmy and non-pygmy groups are available in refs. 18, 20, and 21.
Modeling Fitness.
Hill and Hurtado adapted the Euler–Lotka Equation to include the effect of body size (weight) on fertility (ref. 18; see SI Methods). In our analysis of the Aeta, the equation was rewritten to include the effect of height rather than weight on female fertility as follows:
 |
where lx is survival from birth to age x, α is age at maturity, and mx(Hα) is one-half of the expected fertility at age x (i.e., it only includes number of female offspring) as a function of body size at maturity (Hα). Varying α and solving for r determines which age of maturity corresponds to higher fitness (18). We calculated mx(Hα), or fertility as a function of particular ages associated with attaining adult (reproductive) body height, using logistic regressions that examine the hazards of live births by age and height for parous women (see SI Methods), as follows:
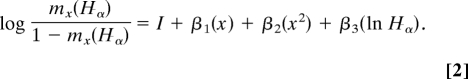 |
Based on the Aeta data, the resulting equation was:
 |
Fertility was then calculated for hypothetical women starting reproduction at different ages [i.e., we calculated mx(Hα) for values of α from 11 to 39]. Size at growth cessation was calculated for one year before birth of the offspring, approximately corresponding to the beginning of pregnancy (see SI Methods). The predicted height (Hα) of hypothetical women stopping growth at different ages at maturity (α) was estimated through an extension of a regression of height on age. The regression was calculated for ages 12 (when the first Aeta girls start reproduction) to 17. The predicted values are shown in SI Table 2. The regression equation is
After calculating mx(Hα) (see SI Table 3) and survivorship to age x (lx), we used Eq. 1 to calculate r (fitness) of hypothetical Aeta women beginning reproduction at different ages and heights. Because r corrects for population growth, it also accounts for the effect of age at first reproduction on generation length of each phenotype (r calculation in SI Methods).
Supplementary Material
ACKNOWLEDGMENTS.
We thank the University of the Philippines, the Philippines National Commission on Indigenous Peoples, L. Dagsaan, J. Eder, R. Foley, T. Headland, K. Hill, V. Paz, and C. Paz, J. Stock, and R. Schlaepfer. We are very grateful to the editor and two anonymous reviewers for useful comments and suggestions. This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, the Parkes Foundation, the Evans Fund, the Crowther-Beynon Fund (A.B.M.), and the Leverhulme Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708024105/DC1.
References
- 1.Cavalli-Sforza LL. African Pygmies. Orlando, FL: Academic; 1986. [Google Scholar]
- 2.Schmidt E. Globus. 1905;87:121–125. [Google Scholar]
- 3.Migliano AB. Cambridge, UK: University of Cambridge; 2005. Thesis. [Google Scholar]
- 4.Turnbull CM. In: African Pygmies. Cavalli-Sforza LL, editor. Orlando, FL: Academic; 1986. pp. 103–123. [Google Scholar]
- 5.Diamond JM. Nature. 1991;354:111–112. doi: 10.1038/354111a0. [DOI] [PubMed] [Google Scholar]
- 6.Biellik RJ, Henderson L. Lancet. 1981;11:1330–1333. doi: 10.1016/s0140-6736(81)91349-0. [DOI] [PubMed] [Google Scholar]
- 7.Little MA, Dyson-Hudson R, Dyson-Hudson N, Winterbauer NL. In: Turkana Herders of the Dry Savannah. Little MA, Leslie PW, editors. Oxford: Oxford Univ Press; 1999. pp. 316–330. [Google Scholar]
- 8.Wheeler EF. In: Human Ecology in Savannah Environments. Harris D, editor. London: Academic; 1980. pp. 439–455. [Google Scholar]
- 9.Brown P, Sutikna T, Morwood MJ, Soejono RP, Jatmiko, Wayhu Saptomo E, Rokus Awe Due. Nature. 2004;431:1055–1061. doi: 10.1038/nature02999. [DOI] [PubMed] [Google Scholar]
- 10.Jacob T, Indriati E, Soejono RP, Hsü K, Frayer DW, Eckhardt RB, Kuperavage AJ, Thorne A, Henneberg M. Proc Natl Acad Sci. 2006;103:13421–13426. doi: 10.1073/pnas.0605563103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falk D, Hildebolt C, Smith K, Morwood MJ, Sutikna T, Brown P, Jatmiko, Saptomo EW, Brunsden B, Prior F. Science. 2007;308:242–245. doi: 10.1126/science.1109727. [DOI] [PubMed] [Google Scholar]
- 12.Eveleth PB, Tanner JM. Worldwide Variation in Human Growth. Cambridge: Cambridge Univ Press; 1991. [Google Scholar]
- 13.van de Koppel JMH, Hewllett BA. In: African Pygmies. Cavalli-Sforza LL, editor. Orlando, FL: Academic; 1986. pp. 103–123. [Google Scholar]
- 14.De Souza RG. Ann Hum Biol. 2007;33:604–619. doi: 10.1080/03014460601062759. [DOI] [PubMed] [Google Scholar]
- 15.National Centre for Health Statistics, U.S. Department of Health and Human Services. National Health Examination Survey (NHANES) 2001–2002. Hyattsville, MD: Centers for Disease Control and Prevention; 2002. [Google Scholar]
- 16.Little MA, Galvin K, Mugambi M. Hum Biol. 1983;55:811–830. [PubMed] [Google Scholar]
- 17.Hill K, Boesch C, Goodall J, Pusey A, Williams J, Wrangham R. Hum Evol. 2001;40:437–450. doi: 10.1006/jhev.2001.0469. [DOI] [PubMed] [Google Scholar]
- 18.Hill KI, Hurtado AM. Ache Life History: The Ecology and Demography of a Foraging People. New York: Aldine de Gruyter; 1996. [Google Scholar]
- 19.Howell N. Demography of the Dobe !Kung. New York: Academic; 1979. [Google Scholar]
- 20.Leslie PW, Dyson-Hudson N, Fry PH. In: Turkana Herders of the Dry Savannah. Little MA, Leslie PW, editors. Oxford: Oxford Univ Press; 1999. pp. 281–301. [Google Scholar]
- 21.Early JD, Headland TN. Population Dynamics of a Philippine Rain Forest People. Gainesville, FL: Univ Press of Florida; 1998. [Google Scholar]
- 22.Weiss KM. Am Antiq. 1973;38:351–381. [Google Scholar]
- 23.Charnov EL. Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology. Oxford: Oxford Univ Press; 1993. [Google Scholar]
- 24.Stearns SC. The Evolution of Life Histories. Oxford: Oxford Univ Press; 1999. [Google Scholar]
- 25.Allal N, Sear R, Prentice A, Mace R. Proc R Soc London Ser B. 2004;271:465–470. doi: 10.1098/rspb.2003.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey PH, Clutton-Brock TH. Evolution (Lawrence, Kans) 1985;39:559–581. doi: 10.1111/j.1558-5646.1985.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 27.Walker R, Gurven M, Hill K, Migliano AB, Chagnon N, De Souza R, Djurovic G, Hames R, Hurtado AM, Kaplan H, et al. Am J Hum Biol. 2006;18:295–311. doi: 10.1002/ajhb.20510. [DOI] [PubMed] [Google Scholar]
- 28.Vinicius L. J Hum Evol. 2005;49:762–776. doi: 10.1016/j.jhevol.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Mirazón Lahr M, Foley RA. Nature. 2004;431:1043–1044. doi: 10.1038/4311043a. [DOI] [PubMed] [Google Scholar]
- 30.Palkovacs EP. Oikos. 2003;103:37–44. [Google Scholar]
- 31.Schebesta P. Die Bambuti Pygmaeen von Ituri. Brussels: Academie Royale des Sciences et Lettres; 1938. [Google Scholar]
- 32.Schebesta P, Lebzelter V. Anthropology of the central African Pygmies in the Belgian Congo. Prague: Académie Tchèque des Sciences et des Arts; 1933. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.