Abstract
The evolution of life history (pace of growth and reproduction) was crucial to ancient hominin adaptations. The study of dental development facilitates assessment of growth and development in fossil hominins with greater precision than other skeletal analyses. During tooth formation, biological rhythms manifest in enamel and dentine, creating a permanent record of growth rate and duration. Quantification of these internal and external incremental features yields developmental benchmarks, including ages at crown completion, tooth eruption, and root completion. Molar eruption is correlated with other aspects of life history. Recent evidence for developmental differences between modern humans and Neanderthals remains ambiguous. By measuring tooth formation in the entire dentition of a juvenile Neanderthal from Scladina, Belgium, we show that most teeth formed over a shorter time than in modern humans and that dental initiation and eruption were relatively advanced. By registering manifestations of stress across the dentition, we are able to present a precise chronology of Neanderthal dental development that differs from modern humans. At 8 years of age at death, this juvenile displays a degree of development comparable with modern human children who are several years older. We suggest that age at death in juvenile Neanderthals should not be assessed by comparison with modern human standards, particularly those derived from populations of European origin. Moreover, evidence from the Scladina juvenile and other similarly aged hominins suggests that a prolonged childhood and slow life history are unique to Homo sapiens.
Keywords: hominin, human evolution, life history, ontogeny, tooth growth
Dental development and life history in Neanderthals (Homo neanderthalensis) have been vigorously discussed over the last 30 years (1–11). Some studies report that Neanderthal dental development is advanced relative to modern and Upper Paleolithic humans (e.g., 1, 3, 4, 6, 9), whereas others show that aspects of Neanderthal development are similar to modern humans (2, 7, 8, 10, 11). Knowledge of the timing of fossil hominin dental development and tooth eruption is fundamental to assessments of growth and development (for review, see refs. 12–14); these former studies often contradict each another by emphasizing similarities or differences in life history among late Pleistocene Homo and recent humans. Moreover, studies of Neanderthal cranial and postcranial ontogeny often use modern European and North American standards of dental development to assign ages and make comparisons (e.g., 4, 15), although it is unclear whether Neanderthal and modern human children actually follow the same schedule of dental development and tooth eruption.
Although some scholars debate whether Neanderthals had shorter periods of anterior tooth growth than modern humans (6, 7), it is known that anterior tooth growth requires more time in great apes than in humans (16) and is variable among human populations (17). This level of variation within and between hominoid species suggests that anterior tooth formation times are not a reliable predictor of life history (6). Furthermore, little is known about ages at tooth eruption in Neanderthals (5), although the timing of molar eruption is the best indicator to predict development and life history parameters across primates (18–21). A recent study reported an age of Neanderthal firstmolar eruption based on an isolated tooth from La Chaise that was similar to the modern human mean value (8). However, this age is difficult to determine from a fully formed and isolated tooth, particularly without knowledge of the mean root length at first molar eruption in other Neanderthals. Additional information regarding dental development and age at molar eruption is necessary to clarify Neanderthal life history relative to modern humans.
Direct assessments of tooth growth are based on incremental features in enamel and dentine, representing consistent short- and long-period biological rhythms that may yield accurate assessments of tooth formation time and age at death (for review, see refs. 12, 22, 23). Resolution of the debate regarding whether Neanderthals and modern humans follow disparate ontogenetic trajectories has been slowed by a lack of data from histological sections of teeth (2). In particular, information on long-period line periodicities (temporal repeat intervals) is lacking (8). Studies of Neanderthal perikymata (the external manifestations of long-period lines) (e.g., 6, 7) offer only a partial solution because their periodicity is typically estimated from mean values of other taxa and may be directly determined only from an internal examination of tooth growth. Before this work, periodicity values from only two sectioned permanent molar teeth have been reported for Neanderthals (8, 10); in neither case was it possible to assess directly the timing of molar eruption or the overall chronology of dental development.
In this work, we report a unique assessment of the timing of tooth formation in a juvenile Neanderthal by using both internal and external developmental features, including the long-period line periodicity and a record of developmental stress over several years that facilitates cross-registry of the dentition. The Scladina cave (Sclayn, Belgium) was discovered in 1971, and since 1993 it has yielded a nearly complete juvenile maxillary and mandibular dentition from a single layer dated to between 80,000 and 127,000 years before present (ypb) (24–27). Previous assessments using a variety of developmental standards have suggested that the juvenile was between 8.5 and 12 years of age at death (27). Here, we directly assess dental development and age at death in the Scladina juvenile. Long-period incremental features on tooth crowns and roots were quantified, the periodicity of long-period lines was determined from an upper first molar, patterns of postnatal developmental stress were calibrated across teeth, and the duration of crown formation and root development was calculated, yielding the age at death. These results were compared with data on living and fossil Homo, including modern humans from Africa and Europe, as well as a recent study of chimpanzee molar formation (8, 10, 16, 17, 28–36).
Results and Discussion
Developmental variables for each tooth are given in supporting information (SI) Table 4. The long-period line periodicity (number of days between long-period lines) was 8 in this individual, which is similar to values of 7 and 8 found for Neanderthals from La Chaise and Tabun, respectively (8). A histological section of the right upper first molar showed a neonatal (birth) line 13 days after mesiopalatal cusp initiation, which allowed subsequent developmental time to be registered to chronological age. Crown formation time of the mesiopalatal cusp was ≈872 days, yielding ages at cuspal crown completion of 2.35 years and 2.22 years for the mesiopalatal and mesiobuccal cusps, respectively. These times are less than mean modern human first molar times (Table 1) but are similar to chimpanzee means (36). Coronal extension rates for the first molar cusps were outside the range of modern human values (Table 2), as were those estimated for a Neanderthal lower first molar from La Chaise (8); Neanderthal first molar crown extension rates also show some similarity to chimpanzee values.
Table 1.
Crown formation times in the Scladina juvenile compared with two modern human populations
| Tooth* | Cusp† | Scladina | North European‡ | N | South African§ | N |
|---|---|---|---|---|---|---|
| UI2 | — | 1,206 | 1,095 ± 49 | 16 | 984 ± 36 | 22 |
| UC | — | 1,301 | 1,398 ± 55 | 39 | 1,213 ± 42 | 30 |
| UM1 | mp | 872 | 1,208 ± 78 | 7 | 1,088 ± 66 | 6 |
| mb | 811¶ | 1,082 ± 47 | 6 | 1,040 ± 64 | 12 | |
| UM2 | mp | 967 | 1,193 ± 49 | 6 | 1,263 ± 82 | 8 |
| mb | 949 | 1,087 ± 59 | 9 | 1,206 ± 74 | 9 | |
| LC | — | 1,326 | 1,866 ± 73 | 13 | 1,494 ± 56 | 25 |
| LP3 | Buccal | 994 | 1,437 ± 68 | 19 | 1,122 ± 42 | 16 |
Values are in days, followed by the standard deviation and sample size (N). Anterior tooth data are from ref. 17, premolar data are from ref. 11, and molar data are from ref. 34.
*U, upper; L, lower; I2, lateral incisor; C, canine; P3, third premolar; M1, first molar; M2, second molar.
†mp, mesiopalatal cusp (protocone); mb, mesiobuccal cusp (paracone). Dash indicates single-cusped tooth.
‡North European indicates modern humans of northern European origin.
§South African indicates modern humans of southern African origin.
¶Assumes an equal amount of prenatal formation as the upper first molar mesiopalatal cusp.
Table 2.
Crown extension rates for human, chimpanzee, and Neanderthal first molars
| Cusp* | Taxon | Mean | N | Range |
|---|---|---|---|---|
| U mp | Homo sapiens | 5.82 | 14 | 5.28–6.66 |
| Pan troglodytes | 7.77 | 3 | 7.66–7.90 | |
| Homo neanderthalensis | 7.60 | 1 | — | |
| U mb | Homo sapiens | 5.87 | 13 | 4.68–7.47 |
| Pan troglodytes | 6.93 | 3 | 5.81–7.61 | |
| Homo neanderthalensis | 8.47† | 1 | — | |
| L mb | Homo sapiens | 6.61 | 18 | 5.35–7.42 |
| Pan troglodytes | 7.94 | 5 | 7.07–8.88 | |
| Homo neanderthalensis | 7.70‡ | 1 | — | |
| L ml | Homo sapiens | 6.30 | 27 | 5.07–7.27 |
| Pan troglodytes | 8.82 | 5 | 8.00–9.43 | |
| Homo neanderthalensis | 7.68‡ | 1 | — |
Values represent the length of the enamel–dentine junction (EDJ) divided by the cusp-specific crown formation time, followed by the sample size of each cusp (N) and the range (minimum to maximum values).
*U, maxillary; mp, mesiopalatal cusp (protocone); mb, mesiobuccal cusp (paracone); L, mandibular; mb, mesiobuccal cusp (protoconid); ml, mesiolingual cusp (metaconid).
†Estimated using minor reconstruction of dentine horn tip and prenatal enamel equal to mesiopalatal cusp.
‡Calculated using mCT data to virtually section tooth (optimizing for mesial EDJ length); cusp-specific EDJ length was divided by formation times reported in ref. 8 for each cusp.
A comparison of formation times across the dentition reveals that certain anterior teeth in the Scladina Neanderthal form over a greater period than modern human population mean times (e.g., upper lateral incisor); however, this finding is not the case for other anterior teeth (e.g., lower canine) or postcanine teeth, which appear to form over shorter periods of time than those from modern human populations (Table 1). Longer formation times in the Scladina anterior teeth relative to modern humans may stem, in part, from the larger size of these teeth in Neanderthals (9). In the case of Neanderthal molars, relatively short formation times may be explained, in part, by differences in cuspal enamel thickness, which are ≈60–90% of modern human mean values (Table 3). Given that overall daily secretion rates do not vary between Neanderthals and modern humans (8, 10), Neanderthal molar cuspal enamel formation times are likely to be shorter than in modern humans. Despite similarities in perikymata numbers (7, 11, 31), this work suggests that Neanderthal postcanine teeth are characterized by shorter periods of overall crown formation than modern humans because of thinner cuspal enamel and faster crown extension rates.
Table 3.
Mean cuspal enamel thickness in modern human and Neanderthal mesial molar cusps
| Tooth* | Cusp† | Human‡ | N | Neanderthal§ | N | % Diff¶ |
|---|---|---|---|---|---|---|
| UM1 | mb | 1,069 ± 169 | 13 | 759 | 2 | 71.0 |
| mp | 1,521 ± 271 | 14 | 954 | 2 | 62.7 | |
| UM2 | mb | 1,322 ± 182 | 23 | 1197 | 3 | 90.5 |
| mp | 1,688 ± 188 | 17 | 1293 | 2 | 76.6 | |
| UM3 | mb | 1,594 ± 276 | 68 | 1338 | 5 | 83.9 |
| mp | 1,855 ± 246 | 55 | 1455 | 5 | 78.4 | |
| LM1 | mb | 1,093 ± 245 | 20 | 866 | 6 | 79.2 |
| ml | 1,061 ± 194 | 27 | 901 | 6 | 84.9 | |
| LM2 | mb | 1,452 ± 148 | 32 | 1060 | 1 | 73.0 |
| ml | 1,209 ± 130 | 25 | 971 | 2 | 80.3 | |
| LM3 | mb | 1,618 ± 247 | 43 | 1006 | 3 | 62.2 |
| ml | 1,342 ± 235 | 30 | 954 | 6 | 71.1 |
*U, maxillary; L, mandibular; M1, first molar; M2, second molar; M3, third molar.
†For maxillary molar cusps: mb, mesiobuccal cusp (paracone); mp, mesiopalatal cusp (protocone); for mandibular molar cusps: mb, mesiobuccal cusp (protoconid); ml, mesiolingual cusp (metaconid).
‡Modern human values were taken from physical sections of mesial cusps of a sample of modern humans (mixed global populations) reported in ref. 34. Thickness measured in micrometers.
§Neanderthal values were taken from virtual sections cut through the mesial cusps of unworn or very lightly worn cusps of molars reported in ref. 37. Thickness measured in micrometers.
¶The percent difference was defined as the Neanderthal mean divided by the human mean, multiplied by 100.
A crucial aspect of registering development across the dentition was the establishment of a chronology of postnatal developmental stress, identified in the enamel and dentine of the first molar at ≈1.2, 2.4, and 4.9 years of age, which was matched across anterior teeth (Fig. 1). Developmental stress at 4.9 years of age in the developing lower third premolar and upper canine was used to cross-match these teeth (SI Fig. 4) and to establish that the individual was ≈8 years old at death (≈2,939 days). This finding was confirmed by counts of long-period lines (Retzius lines and Andresen lines), which were found to be consistent between coronal enamel and dentine in the first molar, as well as between the surfaces of other tooth crowns and roots (represented by perikymata and periradicular bands) (Fig. 1 and SI Figs. 4 and 5). Registry of these features and developmental stress across the dentition allows the establishment of a developmental chronology (Fig. 2), as well as ages at crown initiation and completion (SI Table 5). Tooth crown initiation and completion ages in this juvenile are generally younger than in a modern comparative sample, although some similarities were found with a modern African individual (29).
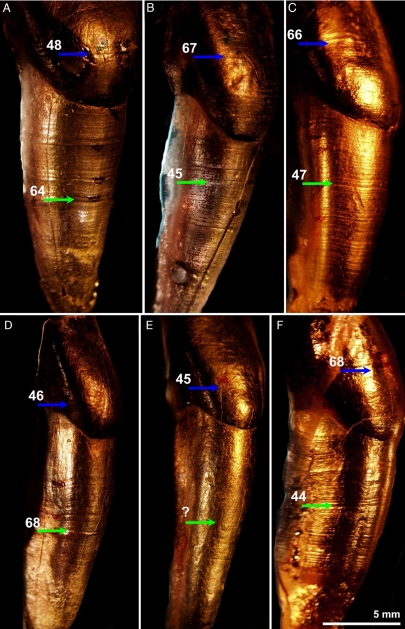
Fig. 1.
Developmental stress matched across the anterior dentition of the Scladina juvenile (gold-coated casts). (A–C) Maxillary dentition: central incisor (A), lateral incisor (B), and canine (C). (D–F) Mandibular dentition: central incisor (D), lateral incisor (E), and canine (F). The first stress event (blue arrow) was identified in the histological section of the upper first molar at 2.4 years of age, with the number of subsequent perikymata after this event indicated on each tooth. The number of periradicular bands between the cervix and subsequent stress event (green arrow) is indicated, with an average of 113 lines between events (904 days). Thus, this second event occurred at 4.9 years of age.
Fig. 2.
Developmental chronology of the Scladina juvenile illustrating crown formation (gray) and root formation (black). The positions of two stress events used to register teeth (see Fig. 1 and SI Fig. 4) are indicated (dotted vertical lines), as is death (solid vertical line). Given that the upper lateral incisor and first molar had completed root formation before death, it was not possible to determine the end of root formation (indicated by question marks).
An examination of modern European tooth calcification standards (28) demonstrates that this individual is ≈2–3 years younger than a modern human juvenile at the same developmental stage and has a particularly advanced degree of third-molar calcification (approximately three-fourths crown complete). Wolpoff (9) noted that third molar calcification in the Krapina Neanderthal sample was 2–3 years ahead of the expected developmental stage of other molar teeth, which also appears to be the case with the Scladina juvenile. This pattern, however, does not appear to be unique to Neanderthals; early third-molar initiation ages have also been reported for modern humans of southern African origin (H. M. Liversidge, unpublished data).
Comparisons of modern human mean tooth eruption ages (32) also confirm that the age at the death in Scladina is several years younger than expected. Although it is not possible to assess the age at first molar eruption in this individual, the advanced attrition on the mesiopalatal cusp of the upper first molar and mesiobuccal cusp of the lower first molar suggests that these teeth have been in functional occlusion for >2 years (the expected mean duration for a modern human juvenile at this age). Furthermore, the first molar roots had reached apical closure before death at 8 years of age, which is younger than the modern human mean or the estimated age for apical closure in the La Chaise Neanderthal (8.7 years) (8). Assuming that upper first molar palatal root completion occurred just before death, the minimum overall rate of palatal root extension is ≈6.6 μm/ day, which is greater than estimated values for modern human lower first molars (6.1 μm/day) and the Neanderthal from La Chaise (6.0 μm/day) (38). Comparison with living and fossil Homo suggests that first molar eruption in the Scladina juvenile was likely to have occurred before 6 years of age, as revealed by heavy attrition, rapid root extension, and the relatively young age of root completion. Furthermore, although first molars are often considered the most reliable predictors of life history, there is a strong correlation of emergence ages between permanent teeth, particularly for adjacent teeth such as first and second molars (18). At 8 years of age, the second molar of Scladina is beyond clinical (gingival) emergence. Second molar eruption occurs on average at 10–13 years of age in global human populations, suggesting that Neanderthals show advanced second molar development relative to modern human populations (also noted in ref. 3).
Among hominoids, which show extended periods of growth and development relative to other primates, living humans are regarded as having the slowest life history, which is particularly evident in their later age at first reproduction and prolonged lifespan (39). Comparisons of the degree of dental development with other similarly aged fossil Homo juveniles show that the Scladina juvenile is less dentally advanced than the Nariokotome Homo erectus male (10), slightly advanced relative to the Obi-Rakhmat Neanderthal (35), and more advanced than the early Homo sapiens juvenile from Jebel Irhoud, Morocco (Fig. 3) (33). The advanced degree of development seen in the H. erectus juvenile has been interpreted to suggest that the modern prolonged period of growth and development was not present in this taxon (10, 12). In contrast, recent data demonstrate a modern human-like pattern of growth and development in a 160,000-ybp H. sapiens juvenile (33). The Scladina juvenile appears to show a developmental pattern intermediate between these two species of Homo. Additional evidence from geographically and temporally disparate Neanderthals, as well as earlier African and European hominins, would shed additional insight into the variation and origins of this developmental pattern.
Fig. 3.
Comparison of the degree of development in the similarly aged fossil juveniles. (A) From Jebel Irhoud (33). (B) From Scladina (right mandible mirrored for comparison). Note the absence of the second molar in the early H. sapiens juvenile and the advanced stage of second molar eruption in the Neanderthal from Scladina.
Evidence from the dentition of the Scladina juvenile suggests that age at death in Neanderthals should not be assessed by comparison with modern human standards, particularly those derived from European or North American populations. Furthermore, given the well established relationship between molar eruption and major events in life history (18–21), this work suggests that the Scladina Neanderthal developed more rapidly than living and fossil H. sapiens, experiencing a shorter childhood and a life history that was accelerated relative to that of H. sapiens. Despite some similarities in brain development and body mass between Neanderthals and Upper Paleolithic and modern humans (40), it appears that Neanderthals are not ontogenetically identical to modern humans, implying disparity in other biological adaptations and aspects of social organization.
Materials and Methods
The dental material recovered to date from the Scladina (Sclayn) Cave in Belgium includes 25 dental elements, representing the entire permanent dentition save for the upper third premolar and also includes three deciduous molars (24–27). Although many of the teeth were found in isolation, they are derived from the same individual as shown by their physical proximity, similar degree of preservation, matching contact facets, precise fit in alveolar sockets, and similar developmental stage (26). This finding was confirmed for the teeth included in this work by matching hypoplastic bands across tooth crowns and roots, which showed identical manifestations of developmental stress (e.g., Fig. 1).
High-resolution peels, molds, and casts of the isolated and erupted (in situ) teeth were made by using Struers' Repliset, Coltene impression materials, and Epo-Tek 301 epoxy resin, respectively. Casts were lightly gold-coated with a sputter coater for visualization with stereomicroscopy. Long-period lines were counted on tooth crowns (perikymata) and roots (periradicular bands) from peels, casts, and original specimens using ×50 or greater magnification, and developmental stress events manifest as hypoplasias were identified and mapped on images of crowns and roots. Given the recent suggestion that periradicular bands may not be equivalent to other long-period lines (41), an experiment was performed with a modern human tooth that demonstrated equivalence between external periradicular bands and internal Andresen lines (SI Fig. 5). Andresen lines have been shown to be equivalent to Retzius lines (42), which are equivalent to perikymata (43), and therefore it is the case that internal and external crown and root long-period lines share the same periodicity.
The teeth were scanned with high-resolution microcomputed tomography (mCT) (Skyscan 1076 and Skyscan 1172) at Antwerp University (Belgium) and the Max Planck Institute for Evolutionary Anthropology (Germany). Cuspal thicknesses for unworn/lightly worn teeth were measured from mCT slices following procedures detailed in ref. 33. A histological section was generated from the upper right first molar (SCLA 4A-4) after casting and mCT scanning. To generate a plane of section passing through the tips of the mesial dentine horns, a virtual model was first generated with Vox Blast Software (Vaytek, Inc.), the tips of the dentine horns were located, and a plane of section was cut through the mesial cusp tips (SI Fig. 6). This virtual model was used to orient the tooth before sectioning, and it facilitated the production of a physical section plane that was very similar to the virtual plane (SI Fig. 7). During this process, the tooth was embedded in methylmethacrylate resin, sectioned with an annular saw (Logitech), and the section was mounted to a microscope slide according to procedures described in refs. 30 and 36. The total tissue loss was <1.5 mm in mesiodistal length. The tooth was subsequently removed from methylmethacrylate with dichloromethane and reconstructed to the approximate dimensions and coloration with a dental restorative (temporary resin acrylic) and dental sticky wax.
Crown formation time was determined from the mesiopalatal cusp (protocone) of the right upper first molar according to the procedure described in ref. 44. The neonatal line was identified, and daily cross-striations were counted and/or measured along a prism track from the neonatal line to an accentuated line to yield formation time in days. The accentuated line was tracked down toward the enamel–dentine junction, and the count was continued along a prism to the next accentuated line. This process was repeated until the enamel cervix was reached. It was not possible to determine formation times for cuspal and imbricational regions of the crown because of the degree of attrition, which prohibited identification of the beginning of imbricational enamel formation (typically identified by the first Retzius line to reach the tooth surface). A particularly marked accentuated line was determined to have been formed at ≈435 days of age (1.2 years) and matched in the mesiobuccal cusp; 47 Retzius lines were counted after this line to yield the age at mesiobuccal cusp completion. Coronal extension rates were calculated by division of the cusp-specific enamel–dentine junction length (in micrometers) by the cusp-specific crown formation time (in days) (23, 36).
Crown formation times were determined for the rest of the unworn/lightly worn teeth by summing an estimate of cuspal formation time with the total number of perikymata multiplied by the periodicity (8 days). Cuspal formation time was calculated by two different methods, and an average value was determined; a minimum estimate was calculated as cuspal thickness divided by modern human mean cuspal daily secretion rate: 3.80 μm/day for anterior teeth (45) and 4.11 μm/day for postcanine teeth (34). Linear cuspal enamel thickness was determined from mCT slices (detailed above); measurements were made from the tip of the dentine horn to the approximate position of the first perikymata at the cusp surface. A maximum cuspal formation time estimate was calculated with regression equations for modern human cuspal enamel formation times for anterior and posterior teeth (10, 45).
Developmental stress was mapped in the enamel and dentine of the first molar, registered to hypoplasias on anterior teeth, and subsequent long-period lines were counted on tooth crowns and roots, yielding a chronology of developmental stress over the first 5 years of development. Ages at crown initiation were determined by subtracting coronal developmental time before the known-age stress events (at 875 or 1,779 days), whereas ages at crown completion were determined by adding coronal developmental time after the known-age stress events. The age at death was determined by adding the root formation time of the third premolar after the event (at 1,779 days) to 1,779 days; this tooth was ideal because it showed excellent preservation of the developing root edge (SI Fig. 8). The age of upper third molar initiation was determined by subtracting crown formation time from the age at death, which was possible because this tooth had yet to complete formation (SI Table 4).
Supplementary Material
ACKNOWLEDGMENTS.
This work was made possible by the tremendous field work done at Scladina Cave by a team led by archaeologist Dominique Bonjean, under the direction of Prof. Marcel Otte of the Department of Prehistory of the University of Liège (Belgium). We thank them for their contribution. Nora De Clerck, Heiko Temming, and Pam Walton also provided helpful technical support; Debbie Guatelli-Steinberg provided comparative information; and Wendy Dirks, Allison Cleveland, and two anonymous reviewers provided comments on the manuscript. We also thank Richard Klein and Paul Tafforeau for assistance and support. This work was supported by European Union FP6 Marie Curie Actions MRTN-CT-2005-019564, the Max Planck Society, the University of Liège, the city of Andenne, and the Direction of Archéologie, Ministère de la Région Wallonne Belgium.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707051104/DC1.
References
- 1.Dean MC, Stringer CB, Bromage TG. Am J Phys Anthropol. 1986;70:301–309. doi: 10.1002/ajpa.1330700305. [DOI] [PubMed] [Google Scholar]
- 2.Stringer CB, Dean MC, Martin RD. In: Primate Life History and Evolution. De Rousseau CJ, editor. New York: Wiley; 1990. pp. 115–152. [Google Scholar]
- 3.Tompkins RL. Am J Phys Anthropol. 1996;99:103–118. doi: 10.1002/(SICI)1096-8644(199601)99:1<103::AID-AJPA6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Thompson JL, Nelson AJ. J Hum Evol. 2000;38:475–495. doi: 10.1006/jhev.1999.0364. [DOI] [PubMed] [Google Scholar]
- 5.Kelley J. Nature. 2004;428:904–905. doi: 10.1038/428904b. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez Rozzi FV, Bermdez de Castro JM. Nature. 2004;428:936–939. doi: 10.1038/nature02428. [DOI] [PubMed] [Google Scholar]
- 7.Guatelli-Steinberg D, Reid DJ, Bishop TA, Larsen CS. Proc Natl Acad Sci USA. 2005;102:14197–14202. doi: 10.1073/pnas.0503108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macchiarelli R, Bondioli L, Debénath A, Mazurier A, Tournepiche J-F, Birch W, Dean C. Nature. 2006;444:748–751. doi: 10.1038/nature05314. [DOI] [PubMed] [Google Scholar]
- 9.Wolpoff MH. Am J Phys Anthropol. 1979;50:67–114. doi: 10.1002/ajpa.1330500110. [DOI] [PubMed] [Google Scholar]
- 10.Dean C, Leakey MG, Reid DJ, Shrenk F, Schwartz GT, Stringer C, Walker A. Nature. 2001;414:628–631. doi: 10.1038/414628a. [DOI] [PubMed] [Google Scholar]
- 11.Guatelli-Steinberg D, Reid DJ. J Hum Evol. 2008 doi: 10.1016/j.jhevol.2007.09.016. in press. [DOI] [PubMed] [Google Scholar]
- 12.Dean MC. Proc R Soc London Ser B. 2006;273:2799–2808. [Google Scholar]
- 13.Smith BH, Tompkins RL. Annu Rev Anthropol. 1995;24:257–279. [Google Scholar]
- 14.Skinner MM, Wood B. In: The Evolution of Human Life History. Hawkes K, Paine RP, editors. Santa Fe, NM: School of American Research Press; 2006. pp. 331–400. [Google Scholar]
- 15.Tillier AM. Anthropologie. 2000;38:109–120. [Google Scholar]
- 16.Dean MC, Reid DJ. Am J Phys Anthropol. 2001;116:209–215. doi: 10.1002/ajpa.1116. [DOI] [PubMed] [Google Scholar]
- 17.Reid DJ, Dean MC. J Hum Evol. 2006;50:329–346. doi: 10.1016/j.jhevol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Smith BH, Crummett TL, Brandt KL. Yrbk Phys Anthropol. 1994;37:177–231. [Google Scholar]
- 19.Smith BH. Evolution (Lawrence Kans) 1989;43:683–688. doi: 10.1111/j.1558-5646.1989.tb04266.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith BH. Am J Phys Anthropol. 1991;86:157–174. [Google Scholar]
- 21.Dirks W, Bowman JE. J Hum Evol. 2007;53:309–320. doi: 10.1016/j.jhevol.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Smith TM. J Anat. 2006;208:99–114. doi: 10.1111/j.1469-7580.2006.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith TM, Reid DJ, Sirianni JE. J Anat. 2006;208:125–138. doi: 10.1111/j.1469-7580.2006.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otte M, Toussaint M, Bonjean D. Bull Mém Soc Anthropol Paris. 1993;5:327–332. [Google Scholar]
- 25.Toussaint M, Otte M, Bonjean D, Bocherens H, Falgueres C, Yokoyama Y. C R Acad Sci. 1998;326:737–742. [Google Scholar]
- 26.Toussaint M, Pirson S, Bocherens H. Anthropol Praehist. 2001;112:21–38. [Google Scholar]
- 27.Toussaint M, Pirson S. Period Biol. 2006;108:373–387. [Google Scholar]
- 28.Smith BH. In: Advances in Dental Anthropology. Kelley MA, Larsen CS, editors. New York: Wiley; 1991. pp. 143–168. [Google Scholar]
- 29.Dean MC, Beynon AD, Reid DJ, Whittaker DK. Int J Osteoarch. 1993;3:249–264. [Google Scholar]
- 30.Reid DJ, Beynon AD, Ramirez Rozzi FV. J Hum Evol. 1998;35:463–477. doi: 10.1006/jhev.1998.0233. [DOI] [PubMed] [Google Scholar]
- 31.Reid DJ, Guatelli-Steinberg D, Walton P. J Hum Evol. 2008 doi: 10.1016/j.jhevol.2007.09.015. in press. [DOI] [PubMed] [Google Scholar]
- 32.Liversidge H. In: Patterns of Growth and Development in the Genus Homo. Thompson JL, Krovitz GE, Nelson AJ, editors. Cambridge, UK: Cambridge Univ Press; 2003. pp. 73–113. [Google Scholar]
- 33.Smith TM, Tafforeau PT, Reid DJ, Grün R, Eggins S, Boutakiout M, Hublin JJ. Proc Natl Acad Sci USA. 2007;104:6128–6133. doi: 10.1073/pnas.0700747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith TM, Reid DJ, Dean MC, Olejniczak AJ, Ferrell RJ, Martin LB. In: Dental Perspectives on Human Evolution: State of the Art Research in Dental Paleoanthropology. Bailey SE, Hublin JJ, editors. Dordrecht: Springer; 2007. pp. 177–192. [Google Scholar]
- 35.Smith TM, Reid DJ, Olejniczak AJ, Bailey S, Glantz M, Viola B, Hublin J-J. In: Neanderthals, Their Ancestors and Contemporaries. Condemi S, Schrenk F, Weniger G, editors. Dordrecht: Springer; in press. [Google Scholar]
- 36.Smith TM, Reid DJ, Dean MC, Olejniczak AJ, Martin LB. J Hum Evol. 2007;52:201–216. doi: 10.1016/j.jhevol.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Olejniczak AJ, Smith TM, Feeney RNM, Macchiarelli R, Mazurier A, Bondioli L, Rosas A, Fortea J, de la Rasilla M, García-Tabernero A, et al. J Hum Evol. 2007 doi: 10.1016/j.jhevol.2007.11.004. in press. [DOI] [PubMed] [Google Scholar]
- 38.Dean MC. J Hum Evol. in press. [Google Scholar]
- 39.Robson SL, van Schaik CP, Hawkes K. In: The Evolution of Human Life History. Hawkes K, Paine RP, editors. Santa Fe, NM: School of American Research Press; 2006. pp. 17–44. [Google Scholar]
- 40.Coqueugniot H, Hublin JJ. Period Biol. 2007;109:379–385. [Google Scholar]
- 41.Dean MC. Proc Natl Acad Sci USA. 2007;104:6093–6094. doi: 10.1073/pnas.0701317104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dean MC, Scandrett AE. Arch Oral Biol. 1996;41:233–241. doi: 10.1016/0003-9969(95)00137-9. [DOI] [PubMed] [Google Scholar]
- 43.Risnes S. Scand J Dent Res. 1985;93:145–152. doi: 10.1111/j.1600-0722.1985.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 44.Boyde A. In: Primate Life History and Evolution. De Rousseau CJ, editor. New York: Wiley–Liss; 1990. pp. 229–267. [Google Scholar]
- 45.Schwartz GT, Dean C. Am J Phys Anthropol. 2001;115:269–283. doi: 10.1002/ajpa.1081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.