Abstract
The Fra3B locus on chromosome 3p14.2 targeting the fragile histidine triad (Fhit) gene represents one of the most common fragile sites of the human genome and is associated with early preneoplastic and malignant disorders in multiple human tumors. Fhit was classified as a tumor suppressor; however, the molecular mechanisms of its function are not well established. Here, we report that Fhit associates with the lymphoid enhancer-binding factor 1/T cell factor/β-catenin complex by directly binding to β-catenin, a major player in the canonical Wnt pathway that is deregulated in numerous forms of human cancer. In binding to the β-catenin C-terminal domain, Fhit represses transcription of target genes such as cyclin D1, axin2, MMP-14, and survivin. Knockdown of Fhit reversed this effect, whereas this reversal was not detectable when β-catenin was knocked down simultaneously. The Fhit enzymatic activity as a diadenosine-polyphosphate hydrolase is not required for the down-regulation of β-catenin-mediated transcription as examined with an enzymatic inactive Fhit-H96N protein. ChIPs revealed recruitment of Fhit/β-catenin complexes to target gene promoters. In soft agar assays Fhit and β-catenin are involved in regulation of anchorage-independent growth. These observations assign to the tumor suppressor Fhit an unexpected role in the regulation of β-catenin-mediated gene transcription.
Keywords: histidine triad, T cell factor, lymphoid enhancer-binding factor 1
The fragile histidine triad (Fhit) gene locus FRA3B spans >1 Mb on the short arm of chromosome 3 and is frequently targeted by genomic rearrangements and epigenetic inactivation in numerous human tumors resulting in impaired or missing expression of the encoded Fhit protein (1). Loss of Fhit expression was reported to represent an early event during multistep carcinogenesis and was correlated with tumor progression and worse prognosis (2, 3). In line with a tumor suppressor function, reexpression of Fhit in multiple Fhit-negative tumor cells resulted in inhibition of tumor growth in nude mouse models and Fhit knockout mice (4). This function was attributed to a proapoptotic activity of Fhit (1), which appears to be mediated by a tyrosine phosphorylation-dependent modulation of the Akt-survivin pathway (5, 6).
The evolutionary conserved Fhit protein is composed of 147 aa and represents the second branch of the histidine triad (HIT) protein superfamily, which is characterized by a His-X-His-X-His-XX (X: hydrophobic amino acid) motif (7). Fhit, like all members of the HIT superfamily, has proven nucleotide-binding and nucleotide-hydrolyzing activity preferentially for di aden osine polyphosphates (ApnA, n = 3 or 4) (8). ApnAs have been identified as intracellular signaling molecules that are synthesized in side reactions of certain tRNA synthetases under stress conditions (9). Site-directed mutagenesis of the central histidine 96 of the HIT to asparagine generates a mutant Fhit protein that although enzymatically dead still shows tumor suppressive and proapoptotic activity (10, 11). This observation indicates that hydrolysis of diadenosine polyphosphates is not involved in the tumor-suppressive function of Fhit. Because this protein is still able to bind diadenosine polyphosphates, it was suggested that binding of dinucleotide polyphosphates is relevant (7, 11, 12).
Interacting with multiple partners at the cell membrane, in the cytosol and in the nucleus, β-catenin is involved in diverse cellular processes affecting morphogenesis, proliferation, differentiation, and apoptosis. Therefore, β-catenin level and function have to be tightly controlled within a cell. Cytoplasmic β-catenin levels are controlled by the β-catenin destruction complex composed of the adenomatous polyposis coli protein APC, Axin, Dishevelled, and the Ser/Thr kinases CK1 and glycogen synthase kinase-3β (GSK3β) as major components. Consecutive phosphorylation by CK1 and GSK3β marks β-catenin for degradation by the ubiquitin-proteasome system. Inhibition of β-catenin phosphorylation either by activation of the Wnt-signaling pathway or by mutations in the APC tumor suppressor or the phosphorylation sites in β-catenin itself results in its accumulation and translocation into the cell nucleus (13). There, β-catenin associates with transcription factors such as lymphoid enhancer-binding factor 1 (LEF-1)/T cell factor (TCF) family members (14), Prop1 (15) or NF-κB (16, 17) and modulates transcription of target genes. However, activation of the Wnt pathway is not the only mechanism to regulate β-catenin transcriptional activity. Activation of receptor tyrosine kinases such as the epidermal growth factor and hepatocyte growth factor (18, 19) receptors induces the release of β-catenin from cadherins and increases signaling activity. Furthermore, Akt/PKB-dependent phosphorylation of β-catenin has been shown to enhance β-catenin transcriptional activity (20).
In addition to the β-catenin destruction complex, proteins involved in the nuclear control of β-catenin activity such as Bcl-9, Pygopus, and Parafibromin (14) are of major importance for the role of β-catenin in development and tumorigenesis. We recently showed that Hint1, a member of the first branch of the HIT family, triggers apoptosis (21) and inhibits β-catenin transcriptional activity, resulting in an inappropriate expression of target genes (22). Based on these observations we hypothesized that the tumor suppressor Fhit, a member of the second branch of HIT proteins, may also modulate β-catenin-mediated gene transcription. Here, we show that, in contrast to Hint1, Fhit directly interacts with β-catenin and negatively regulates transcription of target genes.
Results
Fhit Interacts with β-Catenin in Vitro and in Vivo.
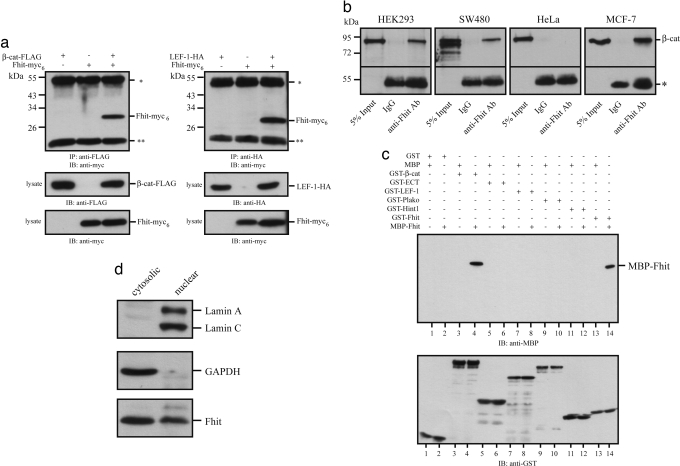
To examine whether Fhit is associated with the LEF-1/β-catenin transcription complex coimmunoprecipitations were performed with lysates obtained from HEK293 cells transiently transfected with Fhit-myc6 and β-catenin-FLAG or LEF-1-HA. Fhit-myc6 was readily detectable in immunoprecipitates of anti-FLAG or anti-HA antibodies (Fig. 1a). Moreover, endogenous Fhit/βcatenin protein complexes were precipitated from HEK293, SW480, and MCF-7 cell lysates with an anti-Fhit antibody and vice versa by an anti-β-catenin antibody but not from lysates of HeLa cells that do not express endogenous Fhit [Fig. 1b and supporting information (SI) Fig. 5a].
Fig. 1.
Fhit associates with β-catenin. (a) HEK293 cells were transiently transfected with FLAG-tagged β-catenin and myc6-tagged Fhit or HA-tagged LEF-1, and after 42 h cells were lysed. Protein complexes were immunoprecipitated with anti-FLAG or anti-HA antibodies, respectively, and subsequently analyzed by Western blotting with anti-myc (9E10) antibody. Lysate controls are shown. (b) Coimmunoprecipitation of endogenous Fhit/β-catenin complexes in HEK293, SW480, and MCF-7 but not in HeLa cells that do not express Fhit. Immunoprecipitations were performed with an anti-Fhit polyclonal antibody. IgG was used as control. (c) Protein complexes were formed with purified recombinant fusion proteins and pulled down with GSH-agarose beads to show a specific direct interaction of MBP-Fhit with GST-β-catenin and GST-Fhit but not with plakoglobin, LEF-1, or the E-cadherin cytoplasmic tail (ECT). By contrast, Hint1, another HIT family protein, does not directly bind to β-catenin. (d) Cytoplasmic and nuclear fractions of MCF-7 cells were analyzed by Western blotting with the indicated antibodies. All data are representative of at least three independent experiments. * indicates heavy chains of the precipitating antibodies, and ** indicates light chains of the precipitating antibodies.
To test whether Fhit directly interacts with LEF-1 or β-catenin, in vitro pull-down experiments with purified recombinant fusion proteins were performed. In contrast to Hint1, Fhit directly associates with β-catenin. However, Fhit does not bind to LEF-1, plakoglobin, Hint1, or the cytoplasmic tail of E-cadherin (Fig. 1c). β-Catenin N- and C-terminal deletion constructs were used to locate the Fhit binding site within amino acids 683–781 of the β-catenin C terminus (SI Fig. 5b), which orchestrates the nuclear effector function of β-catenin by linking β-catenin with the general transcription machinery or chromatin remodeling (14, 23). Conversely, β-catenin binds to amino acids 1–75 in Fhit (SI Fig. 5c). Consistent with structural data (8, 24), dimerization of Fhit involves the C terminus of Fhit. Moreover, we showed that binding of Fhit to β-catenin does not dissociate the β-catenin/LEF-1 complex. Rather a ternary LEF-1/β-catenin/Fhit-complex was formed (SI Fig. 5d), suggesting that Fhit can associate with LEF-1/TCF/β-catenin complexes to modulate transcriptional activity. This function requires nuclear localization of Fhit as analyzed by biochemical fractionation of MCF-7 cells, confirming that a partial fraction of cellular Fhit protein is indeed nuclear (Fig. 1d).
Fhit Represses β-Catenin Transcriptional Activity.
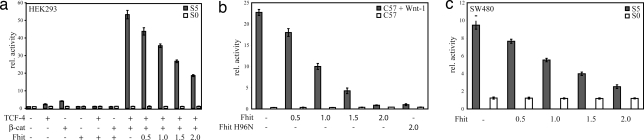
To address whether binding of Fhit to β-catenin modulates β-catenin nuclear function, Siamois-luciferase (25) (Fig. 2a) and pGL3-OT/OF-luciferase (data not shown) reporter gene assays were performed in HEK293 cells. Cotransfection of increasing amounts of Fhit resulted in a dose-dependent repression of TCF/β-catenin-mediated transcription. Transfection of Fhit did not result in detectable changes in β-catenin levels or its localization (data not shown). In contrast, no effects were detected when reporter constructs with mutated LEF-1/TCF binding sites (Siamois S0/pGL3-OF) were used. Moreover, Fhit did not affect CMV promoter-driven β-galactosidase activity used to normalize transfection efficiency (data not shown). To examine whether the repressive effect of Fhit on TCF/β-catenin-mediated transcription depends on β-catenin we performed reporter gene assays with ΔNLEF-VP16 (26), a construct that directly drives transcription independent of β-catenin via the transactivation domain of the herpes simplex virus protein VP16 (SI Fig. 6a), indicating that the binding of Fhit to β-catenin is essential for its repressive function. A LEF-1 construct with deleted β-catenin binding domain also was not affected by Fhit (data not shown).
Fig. 2.
Fhit represses β-catenin transcriptional activity. Increasing amounts of Fhit (0.5, 1.0, 1.5, and 2.0 μg) were transiently cotransfected with Siamois luciferase (S5, five TCF binding sites; S0, all TCF binding sites mutated) (25) together with pCH110 encoding β-galactosidase to normalize transfection efficiency. After 42 h luciferase and β-galactosidase activities were measured in a luminometer. For all experiments mean values of four independent transfections measured in duplicate and ± SEM are presented. (a) S5/S0 reporter gene activity in HEK293 cells cotransfected with different combinations of TCF-4 and β-catenin. (b) C57MG mammary gland cells stably transfected with Wnt-1 and control cells were transiently cotransfected with Siamois luciferase constructs, pCH110, and increasing amounts of Fhit or enzymatic-dead Fhit-H96N (2.0 μg) expression plasmids. (c) SW480 colon carcinoma cells exhibiting high endogenous β-catenin transcriptional activity were transiently transfected with S5/S0 reporter plasmids and increasing amounts of Fhit expression plasmid as indicated.
To further confirm these results, we analyzed β-catenin transcriptional activity in C57MG and NIH 3T3 cells stably transfected with Wnt-1 and compared them with control cells not expressing Wnt-1. In these cells, autocrine activation of the Wnt pathway results in accumulation of endogenous transcriptional active β-catenin. Again, a dose-dependent repressive effect of Fhit was detected in Siamois reporter gene assays (Fig. 2b and SI Fig. 6b). Next, we addressed whether Fhit is able to suppress β-catenin transcriptional activity in SW480 colon carcinoma cells, which express high levels of transcriptional active β-catenin caused by a mutant APC protein, a central component of the β-catenin degradation complex (27). Transfection of Fhit similarly repressed reporter gene activity (Fig. 2c). Cyclin D1 promoter–luciferase constructs revealed that a negative effect of Fhit is also detectable on another target gene promoter (28) (SI Fig. 6c).
Previously, it was reported that Fhit enzymatic activity is not required for its tumor-suppressive function (10). Therefore, we asked whether the diadenosine–polyphosphate hydrolase activity of Fhit is necessary for the repression of β-catenin-mediated transactivation. A hydrolase-dead Fhit-H96N protein with the central histidine in the HIT mutated to asparagine revealed comparable repressive effects as detected for wild-type Fhit (Fig. 2b and SI Fig. 6b).
In a next step, endogenous expression of well established target genes of the TCF/β-catenin transcription complex, such as cyclin D1 (28, 29), axin2 (30), MMP-14 (31), and survivin (32), were examined in response to Fhit. Cyclin D1 mRNA and protein levels were reduced in SW480 or MCF-7 cells after transient transfection with Fhit, as analyzed by real-time RT-PCR and Western blotting. Similarly, axin2, MMP-14, and survivin mRNA levels were reduced in both cell lines (SI Fig. 7 a and b). From these observations, we concluded that in response to loss or reduction of Fhit cells enhance TCF/β-catenin transcriptional activity.
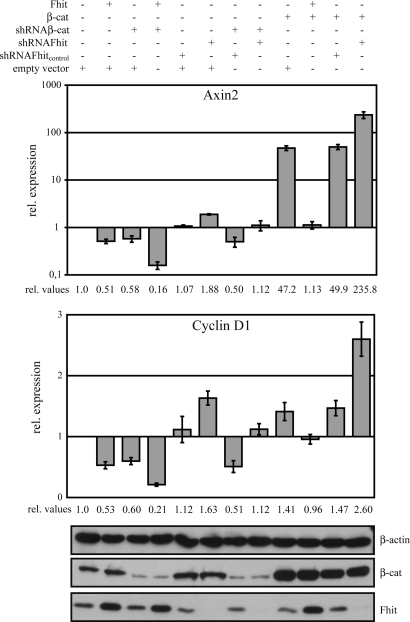
To investigate this and the interplay of Fhit and β-catenin in more detail, MCF-7 cells were transiently transfected with different combinations of Fhit and β-catenin expression plasmids and Fhit and β-catenin shRNA constructs and expression of target genes was quantified by real-time RT-PCR. The levels of β-catenin and Fhit in these transient transfection assays were analyzed by Western blotting. Indeed, transfection of shFhit enhanced target gene expression, whereas shFhitcontrol did not show an effect (Fig. 3 and SI Fig. 7 c and d). Fhit together with shβ-catenin resulted in further down-regulation compared with Fhit expression alone. Because shβ-catenin did not result in a complete knockdown of β-catenin protein, shβ-catenin always enhanced Fhit-induced effects and reverted effects of shFhit but did not show complete null phenotypes. As expected, overexpression of β-catenin increased target gene expression, which was further enhanced in the presence of shFhit, whereas β-catenin in the presence of shFhitcontrol did not show this effect. Comparable changes were observed for cyclin D1, MMP-14, and survivin expression. In respect to axin2 overexpression of β-catenin +/− shFhit revealed dramatically stronger effects compared with the other target genes, resulting in an ≈50-fold increase in response to β-catenin and ≈235-fold increase in response to β-catenin + shFhit (Fig. 3 and SI Fig. 7d). Taken together, these data indicate that genetically Fhit acts upstream or at the level of β-catenin to modulate its nuclear function.
Fig. 3.
Fhit regulates endogenous axin2 and cyclin D1 expression. MCF-7 cells were transiently transfected with different combinations of the indicated Fhit, β-catenin, shFhit, and shβ-catenin constructs. After 42 h total RNA was isolated to analyze axin2 and cyclin D1 expression by quantitative RT-PCR. Expression was normalized to β-actin. Note that the data for axin2 are presented at a logarithmic scale. Bars represent fold induction of expression relative to mock-transfected cells. All data represent experiments that were performed four times.
Fhit and β-Catenin Form Complexes at Promoters of Target Genes and Regulate Growth.
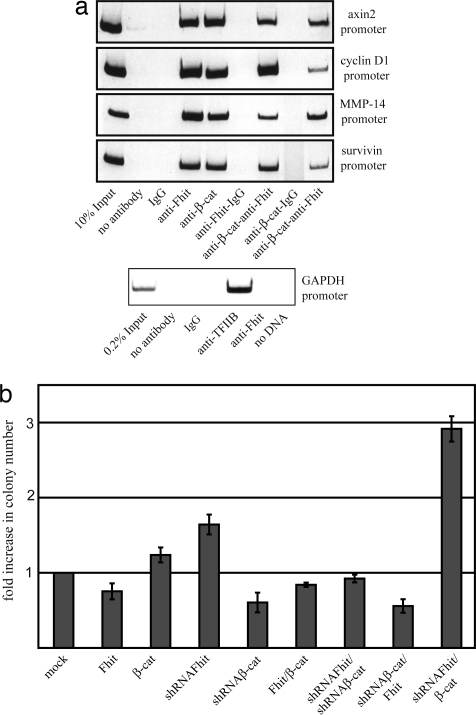
In this context, we next examined whether Fhit is recruited to promoters of TCF/β-catenin target genes. Indeed, in ChIP experiments Fhit and β-catenin were detected at the axin2, cyclin D1, MMP14, and survivin promoters in MCF-7 cells. By contrast, Fhit was not associated with the GAPDH promoter in ChIPs with anti-TFIID antibody showing that Fhit is not a general gene regulatory factor. To prove that Fhit is recruited to these promoters in complexes with β-catenin two-step ChIP assays were performed and we found that Fhit and β-catenin are associated in a complex at these promoters (Fig. 4a).
Fig. 4.
Fhit and β-catenin are recruited to cyclin D1, axin2, MMP-14, and survivin promoters in MCF-7 cells and regulate growth. (a) ChIP and two-step ChIP analyses were performed by using anti-Fhit and anti-β-catenin antibodies in the indicated order. IgG was used as a control. Fhit is not associated with the GAPDH promoter as a general transcription factor as shown by ChIP with anti-TFIIB. (b) Anchorage-independent growth in soft agar of MCF-7 cells transiently transfected with the indicated constructs. Numbers summarize results of four experiments.
To determine the cellular role of Fhit and β-catenin under a physiologically relevant setting, anchorage-independent growth of MCF-7 cells after transient transfection of Fhit and/or β-catenin expression or shRNA constructs was examined. Overexpression of Fhit reduced colony number, whereas β-catenin enhanced colony formation. Knockdown of Fhit or β-catenin induced opposite effects. Both cotransfection of β-catenin and Fhit or β-catenin and Fhit shRNA constructs resulted in a reduction of colony numbers compared with control cells, indicating that enhanced growth resulting from overexpression of β-catenin can be reversed by an increase in Fhit and vice versa. Overexpression of Fhit in a β-catenin knockdown situation did not result in a significant further reduction of colonies compared with the β-catenin knockdown cells. Intriguingly, knockdown of Fhit and concomitant overexpression of β-catenin resulted in strongly enhanced growth properties of cells. Taken together, these data suggest that Fhit by its interaction with β-catenin modulates growth and the transforming potential of β-catenin (Fig. 4b and SI Fig. 8).
Discussion
During recent years a number of new nuclear factors have been identified that associate with nuclear β-catenin, which acts as a molecular interaction hub (33) at target gene promoters. Previous protein-chemical studies analyzing proteins associated with the β-catenin C terminus, including Arm-repeats 11 and 12, have not identified Fhit, probably because of its low molecular weight that made it difficult to detect (34). The assembly of context-dependent transcription complexes acting as specific transcription modules regulate the signaling output induced by different signals such as Wnts (14), EGF and hepatocyte growth factor, or activated Akt/PKB (20). In this respect, different factors such as Bcl-9, Pygopus, Mediator, Parafibromin, CBP/p300, Pontin, and Reptin (14) have been identified as nuclear β-catenin binding partners. Moreover, we recently showed that the HIT protein Hint1 negatively regulates β-catenin-dependent transcription (21, 22). Here, we show that Fhit, another member of the HIT protein family, also inhibits β-catenin nuclear activity, however, by a different mechanism in directly binding to the C terminus of β-catenin. There, Fhit may compete with other regulatory factors such as CBP/p300 (35) or parafibromin (33) and inhibit their transcriptional activator function. Consistent with previous reports, the enzymatic activity of Fhit is not required for its tumor-suppressive activity. However, because Fhit-H96N still binds diadenosine polyphosphates (11) and Fhit mutants that do not bind nucleotides have not been identified up to now, it is currently not clear whether binding of diadenosine polyphosphates affects the interaction of Fhit with β-catenin. Because Fhit does not repress transcriptional activation induced by a LEF-VP16 construct, binding of Fhit to β-catenin appears to be essential. Consistent with this observation, knockdown of Fhit enhances TCF/β-catenin-mediated transcription, whereas after concomitant knockdown of β-catenin this effect was reversed.
Currently, it cannot be excluded that Fhit may also have an adaptor function bridging β-catenin to other factors modulating β-catenin transcriptional activity. There is evidence that Fhit most likely is not a component dedicated solely to the Wnt pathway but also is involved in other signaling pathways. In this respect, it was reported that Fhit inhibits the NF-κB signaling pathway (36) and targets multiple components of Ras/Rho GTPase signaling (37). In addition, Fhit is a target of Src (5), and Src-mediated phosphorylation of Fhit in response to EGF receptor stimulation induces proteasomal degradation of Fhit (38). Furthermore, Fhit has been shown to decrease survivin expression and modulate the Akt-survivin pathway (6). Consistent with this observation, we showed that Fhit contributes to a reduced expression of survivin at the mRNA level in SW480 and MCF-7 cells. These data are in line with previous observations showing that survivin expression is TCF/β-catenin-dependent at least in part (32, 39).
In summary, our results provide a molecular mechanism showing how Fhit can act as a tumor suppressor. Loss of functional Fhit acting as a negative regulator of β-catenin-mediated transcription will contribute to enhanced anchorage-independent growth and thus augment transforming potential induced by deregulated β-catenin signaling, which may explain the worse prognosis observed in many Fhit-negative human tumors.
Materials and Methods
Cell Culture and Antibodies.
HEK293, SW480, HeLa, MCF-7, C57MG, and NIH 3T3 cells were grown in supplemented DMEM as reported (21). Monoclonal anti-FLAG M2, anti-maltose-binding protein (MBP) (clone MBP-17), anti-α-actinin (clone BM-75.2), and anti-β-actin (clone AC15) antibodies were purchased from Sigma. Anti-Cyclin D1 (clone Ab-3) was from Calbiochem, anti-HA (clone 12CA5) was from Roche, anti-β-catenin (clone 14) was from BD Biosciences, and anti-Fhit was from Upstate Biotechnology. HRP-labeled goat anti-mouse and anti-rabbit antibodies were purchased from Dianova.
Plasmids.
Human Fhit cDNA was amplified from total RNA of SW480 cells by RT-PCR using oligonucleotides 5′-CGC GGA TCC GCC ACC ATG TCG TTC AGA TTT GGC CAA-3′ and 5′-CGC GGA TCC TCA CTG AAA GTA GAC CCG CAG-3′. The PCR product was cloned into plasmids pFLAG-CMV4 (Sigma), pGEX-4T1, pMal (NEB), and pQE40 (Qiagen). A myc-tagged variant of Fhit was generated by cloning into pCS2+myc6. Deletion constructs of Fhit encoding amino acids 1–75 and 74–147 were generated by PCR. Site-directed mutagenesis to generate Fhit-H96N was performed with the QuikChange site-directed mutagenesis kit (Stratagene). Sequences of all constructs were confirmed by resequencing.
Hairpin oligonucleotides to suppress Fhit and β-catenin expression were cloned into the vector pRNAT-H1.1/Neo (GenScript). Oligonucleotide sequences were: Fhit, shRNA, 5′-GAT CCC CTA GGA AAC CTG TGG TAC CAT TCA AGA GAT GGT ACC ACA GGT TTC CTA TTT TTG GAA A-3′ and 5′-AGC TTT TCC AAA AAT AGG AAA CCT GTG GTA CCA TCT CTT GAA TGG TAC CAC AGG TTT CCT AGG G-3′; shRNA, 5′-GAT CCC CTA GGA AAC CTG TGG TAC CAT TCA AGA GAT GGT GCT ATA GGT TTC CTG TTT TTG GAA A-3′, and 5′-AGC TTT TCC AAA AAC AGG AAA CCT ATA GCA CCA TCT CTT GAA TGG TAC CAC AGG TTT CCT AGG G-3′; shRNA-β-catenin, 5′-GAT CCG TCC TGT GTG AGT GGG AGT AGG TGT GCT GTC CCT GTT CCC ACT CAT ACA GGA CTT TTT TGG AAA-3′ and 5′-AGC TTT TCC AAA AAA GTC CTG TAT GAG TGG GAA CAG GGA CAG CAC ACC TAC TCC CAC TCA CAC AGG ACG-3′. Interfering activity of these oligonucleotides was tested in a reporter assay using pJOsiCheck, an improved variant of psiCheck (Promega). Plasmids encoding GST-β-catenin deletion constructs and GST-LEF1 have been described (40).
Transient Transfections, Reporter Gene Assays, and Real-Time RT-PCR.
Transient transfections and reporter gene assays were performed as described (22). For reporter gene assays in HEK293 cells 1 μg of the Siamois-luciferase construct (S5, S0), 0.5 μg of hTCF4, and 0.5 μg of pCS2+β-catenin were transfected. Transfection efficiency was normalized by cotransfection of 0.1 μg of pCH110 (β-gal), and Luciferase activity was measured 42 h after transfection. For quantitative RT-PCR, MCF-7 cells were transiently transfected with Fugene HD (Roche) in total 4 μg of the indicated plasmids (2 μg each or filled up with empty vector).
Total RNA and protein were isolated by using the NucleoSpin RNA/protein kit (Macherey & Nagel), and reverse transcription was performed with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real-time PCR was performed by using the TaqMan Gene Expression assays HS00610344_m1 (axin2), HS00277039_m1 (cyclin D1), HS01037009_g1 (MMP14), and HS00153353_ml (survivin) with unlabeled primers and FAM dye-labeled MGB probes. Human β-actin (unlabeled primer, VIC/TAMRA dye-labeled probe) (Applied Biosystems) served as endogenous control and was not affected under experimental conditions. Each PCR was set up in duplicates, and threshold cycle (Ct) values of the target genes were normalized to the endogenous control. Differential expression was calculated according to the 2−ΔΔCt method (41).
Immunoprecipitation and Western Blot Analysis.
For immunoprecipitation HEK293 cells were transiently transfected with pCS2+Fhit-myc6 (2 μg), pCIneo-LEF-HA (3 μg), and pCS2+β-catenin-FLAG (3 μg). After 48 h cells were incubated with ice-cold lysis buffer [20 mM imidazole (pH 8.0), 150 mM NaCl, 2 mM MgCl2, 300 mM sucrose, 0.25% (vol/vol) Triton X-100 and Complete protease inhibitor mix (Roche)] for 10 min at 4°C. Immunoprecipitation from precleared cell lysates (100 μg of total protein) was performed with 2 μg of the appropriate antibody prebound to Protein-A Sepharose beads as described (42). For detection of endogenous protein complexes, HEK293 cells were lyzed with 20 mM imidazole (pH 6.8), 100 mM NaCl, 2 mM MgCl2, 300 mM sucrose, 10 mM EGTA, 0.1% (vol/vol) Triton X-100, and Complete protease inhibitor mix, and immunoprecipitations were performed with precleared cell lysates (300 μg of total protein) by using 5 μg of anti-β-catenin or anti-Fhit antibody as described. Western blot analysis was performed as described (42). Antibodies were diluted in TST [1.5 μg/ml anti-FLAG M2, 0.5 μg/ml anti-GST, 0.4 μg/ml anti-HA (clone 12CA5), 0.5 μg/ml anti-Cyclin D1 (clone Ab-3), 0.25 μg/ml anti-Fhit, 0.1 μg/ml anti-β-catenin]. Anti-myc (9E10) and anti-MBP (clone MBP-17) antibodies were diluted 1:1,000, and anti-α-actinin (clone AT6/172) was diluted 1:4,000.
Pull-Down and Competition Assays.
Fusion proteins (GST, MBP, or His6) were expressed in Escherichia coli and affinity-purified on glutathione (GSH)-agarose (Sigma), amylose (NEB), or Ni-NTA beads. For pull-down assays, 5 μg of GST or GST-fusion proteins were incubated with 5 μg of MBP-fusion proteins in pull-down buffer [150 mM NaCl, 40 mM imidazole (pH 8.0), 2 mM MgCl2, 300 mM sucrose, 0.25% (vol/vol) Triton X-100] for 30 min at 4°C. Assays were performed as described (22). For competition assays GST-LEF-1 (40) was incubated with β-catenin-His6 for 30 min at 4°C in competition buffer C [150 mM NaCl, 40 mM imidazole (pH 8.0), 2 mM MgCl2, 300 mM sucrose, 0.1% (vol/vol) Triton X-100]. After centrifugation (5 min, 4°C, 20,800 × g), the supernatants were incubated with 35 μl of GSH-agarose beads for 30 min at 4°C. After three washes with buffer C, Fhit-His6 protein was added to the complexes and incubated for 30 min at 4°C. GSH beads were again pelleted by centrifugation, washed three times with buffer C, and resuspended in 2× SDS sample buffer.
ChIP.
The ChIP analysis was performed as described (21). For immunoprecipitation 2.5 μg of anti-Fhit and 2.5 μg of anti-β-catenin antibodies were used. For PCR analysis, 2 μl of the extracted DNA was used as a template for 25–35 cycles of amplification. The following primers were used: cyclin D1 promoter, 5′-CCT CCC GCT CCC ATT CTC TGC CG-3′ (forward), 5′-CCT CGC CGG AGC GTG CGG ACT CTG-3′ (reverse); axin2 promoter, 5′-GGT TTC GCC ATA TTA GGC AGG CTG-3′ (forward), 5′-TCT GCT GCT GCT ATA GAA TGA TTC-3′ (reverse); MMP-14 promoter, 5′-ACC TGG AGG CTC CAC AGG ACC-3′ (forward), 5′-GGG TGG ACA GAA ATT AGG TCA G-3′ (reverse); survivin promoter, 5′-GGG GCG CTA GGT GTG GG-3′ (forward), 5′-TTC AAA TCT GGC GGT TAA TGG C-3′(reverse). PCR products were analyzed on 8% (wt/vol) polyacrylamide gels.
Colony Formation.
Colony formation assays in soft agar were performed essentially as described (43). MCF-7 cells were transiently transfected with the indicated constructs by using Fugene HD, and after 48 h, 2.5 × 103 single cells were plated in 0.3% agar medium. After 3 weeks, plates were fixed with methanol and stained with crystal violet. Plates were scanned, and subsequently colonies were analyzed by using Clono-Counter software (44).
Supplementary Material
ACKNOWLEDGMENTS.
We thank Drs. P. Knaus and R. Tauber for critically reading the manuscript; Dr. D. Kimelman (University of Washington, Seattle) for Siamois reporter plasmids; Dr. O. Tetsu (University of California, San Francisco) for Cyclin D1 reporter plasmids; and Dr. B. Vogelstein (Johns Hopkins University, Baltimore) for pGL3-OT and pGL3–OF. This work was supported by Deutsche Forschungsgemeinschaft Grant HU 881/5-1 (to O.H.), the Charité–Universitätsmedizin Berlin, and the Sonnenfeld-Stiftung.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703664105/DC1.
References
- 1.Huebner K, Croce CM. Nat Rev Cancer. 2001;1:214–221. doi: 10.1038/35106058. [DOI] [PubMed] [Google Scholar]
- 2.Ishii H, Ozawa K, Furukawa Y. J Exp Ther Oncol. 2003;3:291–296. doi: 10.1111/j.1533-869x.2003.01101.x. [DOI] [PubMed] [Google Scholar]
- 3.Huebner K, Croce CM. Br J Cancer. 2003;88:1501–1506. doi: 10.1038/sj.bjc.6600937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pekarsky Y, Zanesi N, Palamarchuk A, Huebner K, Croce CM. Lancet Oncol. 2002;3:748–754. doi: 10.1016/s1470-2045(02)00931-2. [DOI] [PubMed] [Google Scholar]
- 5.Pekarsky Y, Garrison PN, Palamarchuk A, Zanesi N, Aqueilan RI, Huebner K, Barnes LD, Croce CM. Proc Natl Acad Sci USA. 2004;101:3775–3779. doi: 10.1073/pnas.0400481101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semba S, Trapasso F, Fabbri M, McCorkell KA, Volinia S, Druck T, Iliopoulos D, Pekarsky Y, Ishii H, Garrison PN, et al. Oncogene. 2006;25:2860–2872. doi: 10.1038/sj.onc.1209323. [DOI] [PubMed] [Google Scholar]
- 7.Brenner C. Biochemistry. 2002;41:9003–9014. doi: 10.1021/bi025942q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pace HC, Garrison PN, Robinson AK, Barnes LD, Draganescu A, Rösler A, Blackburn GM, Siprashvili Z, Croce CM, Huebner K. Proc Natl Acad Sci USA. 1998;95:5484–5489. doi: 10.1073/pnas.95.10.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y-N, Nechushtan H, Figov N, Razin E. Immunity. 2004;20:145–151. doi: 10.1016/s1074-7613(04)00020-2. [DOI] [PubMed] [Google Scholar]
- 10.Siprashvili Z, Sozzi G, Barnes LD, McCue P, Robinson AK, Eryomin V, Sard L, Tagliabue E, Greco A, Fusetti L, et al. Proc Natl Acad Sci USA. 1997;94:13771–13776. doi: 10.1073/pnas.94.25.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trapasso F, Krakowiak A, Cesari R, Arkles J, Yendamuri S, Ishii H, Vecchione A, Kuroki T, Bieganowski P, Pace HC, et al. Proc Natl Acad Sci USA. 2003;100:1592–1597. doi: 10.1073/pnas.0437915100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenner C, Bieganowski P, Pace HC, Huebner K. J Cell Physiol. 1999;181:179–187. doi: 10.1002/(SICI)1097-4652(199911)181:2<179::AID-JCP1>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giles RH, van Es JH, Clevers H. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 14.Städeli R, Hoffmans R, Basler K. Curr Biol. 2006;16:R378–R385. doi: 10.1016/j.cub.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, et al. Cell. 2006;125:593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 16.Deng J, Miller SA, Wang HY, Xia W, Wen Y, Zhou BP, Li Y, Lin SY, Hung MC. Cancer Cell. 2002;2:323–334. doi: 10.1016/s1535-6108(02)00154-x. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Kim B, Cai L, Choi HJ, Ohgi KA, Tran C, Chen C, Chung CH, Huber O, Rose DW, et al. Nature. 2005;434:921–926. doi: 10.1038/nature03452. [DOI] [PubMed] [Google Scholar]
- 18.Lu Z, Ghosh S, Wang Z, Hunter T. Cancer Cell. 2003;4:499–515. doi: 10.1016/s1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]
- 19.Müller T, Bain G, Wang X, Papkoff J. Exp Cell Res. 2002;280:119–133. doi: 10.1006/excr.2002.5630. [DOI] [PubMed] [Google Scholar]
- 20.Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiske J, Huber O. J Biol Chem. 2006;281:27356–27366. doi: 10.1074/jbc.M513452200. [DOI] [PubMed] [Google Scholar]
- 22.Weiske J, Huber O. J Cell Sci. 2005;118:3117–3129. doi: 10.1242/jcs.02437. [DOI] [PubMed] [Google Scholar]
- 23.Hecht A, Kemler R. EMBO Rep. 2000;1:24–28. doi: 10.1093/embo-reports/kvd012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lima CD, Klein MG, Hendrickson WA. Science. 1997;278:286–290. doi: 10.1126/science.278.5336.286. [DOI] [PubMed] [Google Scholar]
- 25.Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D. Genes Dev. 1997;11:2359–2370. doi: 10.1101/gad.11.18.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aoki M, Hecht A, Kruse U, Kemler R, Vogt PK. Proc Natl Acad Sci USA. 1999;96:139–144. doi: 10.1073/pnas.96.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 28.Tetsu O, McCormick F. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 29.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi M, Tsunoda T, Seiki M, Nakamura Y, Furukawa Y. Oncogene. 2002;21:5861–5867. doi: 10.1038/sj.onc.1205755. [DOI] [PubMed] [Google Scholar]
- 32.Kim PJ, Plescia J, Clevers H, Fearon ER, Altieri DC. Lancet. 2003;362:205–209. doi: 10.1016/S0140-6736(03)13910-4. [DOI] [PubMed] [Google Scholar]
- 33.Mosimann C, Hausmann G, Basler K. Cell. 2006;125:327–341. doi: 10.1016/j.cell.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 34.Sierra J, Yoshida T, Joazeiro CA, Jones KA. Genes Dev. 2006;20:586–600. doi: 10.1101/gad.1385806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hecht A, Vleminckx K, Stemmler MP, van Roy F, Kemler R. EMBO J. 2000;19:1839–1850. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakagawa Y, Akao Y. Exp Cell Res. 2006;312:2433–2442. doi: 10.1016/j.yexcr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Jayachandran G, Sazaki J, Nishizaki M, Xu K, Girard L, Minna JD, Roth JA, Jin J. Cancer Res. 2007;67:10379–10388. doi: 10.1158/0008-5472.CAN-07-0677. [DOI] [PubMed] [Google Scholar]
- 38.Bianchi F, Magnifico A, Olgiati C, Zanesi N, Pekarsky Y, Tagliabue E, Croce CM, Ménard S, Campiglio M. Proc Natl Acad Sci USA. 2006;103:18981–18986. doi: 10.1073/pnas.0605821103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma H, Nguyen C, Lee K-S, Kahn M. Oncogene. 2005;24:3619–3631. doi: 10.1038/sj.onc.1208433. [DOI] [PubMed] [Google Scholar]
- 40.Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R. Mech Dev. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- 41.Livak KJ, Schmittgen TD. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 42.Weiske J, Schöneberg T, Schröder W, Hatzfeld M, Tauber R, Huber O. J Biol Chem. 2001;276:41175–41181. doi: 10.1074/jbc.M105769200. [DOI] [PubMed] [Google Scholar]
- 43.Kolligs FT, Hu B, Dang CV, Fearon ER. Mol Cell Biol. 1999;19:5696–5706. doi: 10.1128/mcb.19.8.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niyazi M, Niyazi I, Belka C. Radiat Oncol. 2007;2:1–3. doi: 10.1186/1748-717X-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.