Abstract
The conserved Drosophila tumor suppressors Fat and Expanded have both recently been implicated in regulating the activity of the Warts tumor suppressor. However, there has been disagreement as to the nature of the links among Fat, Expanded, and Warts and the significance of these links to growth control. We report here that mutations in either expanded or fat can be rescued to viability simply by overexpressing Warts, indicating that their essential function is their influence on Warts rather than reported effects on endocytosis or other pathways. These rescue experiments also separate the transcriptional from the planar cell polarity branches of Fat signaling and reveal that Expanded does not directly affect polarity. We also investigate the relationship between expanded and fat and show, contrary to prior reports, that they have additive effects on imaginal disk growth and development. Although mutation of fat can cause partial loss of Expanded protein from the membrane, mutation of fat promotes growth even when Expanded is overexpressed and accumulates at its normal subapical location. These observations argue against recent proposals that Fat acts simply as a receptor for the Hippo signaling pathway and instead support the proposal that Fat and Expanded can act in parallel to regulate Warts through distinct mechanisms.
Keywords: Drosophila, Hippo, protocadherin
The regulation of growth is of fundamental importance to both normal development and tumor formation. Studies over the last several years have identified a cassette of genes, referred to as the Hippo signaling pathway, that act together to regulate growth (1, 2). Most components were first identified by genetic studies in Drosophila, although there is increasing evidence that homologous genes regulate growth in mammals and that their dysregulation is linked to cancer (1, 2). The eponymous hippo gene (hpo) encodes a kinase that promotes the activation of another kinase, Warts (Wts) (1, 2). Activation of Wts also depends on the Hpo-interacting protein Salvador (Sav) and the Wts-interacting protein Mob as Tumor Suppressor (Mats). Hpo, Wts, Sav, and Mats all act as tumor suppressors. A transcriptional coactivator protein, Yorkie, is negatively regulated by Wts-dependent phosphorylation, and this regulation of Yorkie appears to be sufficient to explain the influence of Wts on growth (3).
Another Drosophila tumor suppressor, expanded (ex), has also been linked to Hippo signaling (4–9). Ex and a structurally related protein, Merlin (Mer), both contain FERM domains, which places them within a family of proteins that link membrane proteins to the cytoskeleton (10). Although Mer has only minor effects on growth in Drosophila, its human homolog acts as a tumor suppressor (10), and genetic studies suggest that Mer and ex are partially redundant (11). Cells doubly mutant for ex and Mer exhibit phenotypes similar to that of other components of the Hippo pathway, and Ex and Mer can influence Hpo and Wts phosphorylation in cultured cells (4–8). Although the recent linkage of Ex and Mer to Hippo signaling in Drosophila suggests that this might account for their influence on growth, it has also been suggested that Ex and Mer influence growth in Drosophila by affecting the endocytosis of multiple cell surface receptors and the activity of multiple signaling pathways (9, 12).
Because Ex is a cytoplasmic protein, the linkage of Ex to Hippo signaling left unanswered the question of whether there are extracellular signals that modulate Hippo pathway activity and, if so, how those signals are received and transduced. Recently it was proposed that the protocadherin Fat acts as a receptor for Hippo signaling (4, 5, 8, 9). Fat is also a Drosophila tumor suppressor and regulates the same downstream genes as are regulated by Hippo signaling (4, 5, 7, 8). Although this could be consistent with Fat acting as a receptor for Hippo signaling, our own studies have identified a distinct, parallel signal transduction pathway in which Fat acts through the unconventional myosin Dachs to regulate the levels of Wts (7).
Thus, prior studies have raised fundamental questions about both the contribution of Ex to Fat signaling and the contribution of Hippo signaling to Ex function. Results we describe here support a model in which Ex acts through the regulation of Wts activity and in so doing acts in parallel to a regulation of Wts protein levels exerted by Fat.
Results
fat or ex Mutants Can Be Partially Rescued by Wts Overexpression.
Although it has been proposed that fat and ex both act through Wts to regulate growth and gene expression, other models have been put forward. For example, it has been suggested that Ex and Mer influence endocytosis and thereby influence growth by modulating the EGFR, Notch, Hedgehog, Wg, and/or Fat signaling pathways (12). It has also been suggested that Fat and Ex influence membrane sterol composition and thereby affect multiple pathways including Wg, Dpp, and Hippo (9).
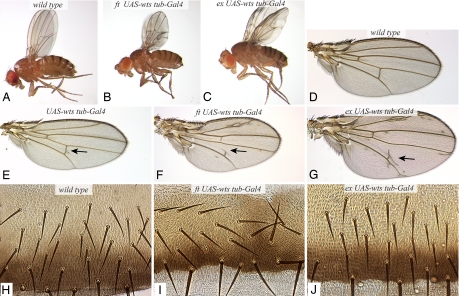
To the extent that Fat signaling involves an influence on Wts stability, we reasoned that it might be possible to saturate this mechanism by overexpressing Wts. Overexpression of Wts did not preclude observation of the influence of Fat signaling on Wts protein levels in prior experiments (7), implying that levels of Wts were not high enough to saturate effects on Wts stability. However, expression of Wts at a higher level, using a different UAS-Myc:Wts transgene insertion [supporting information (SI) Fig. 5], was sufficient to rescue fat mutant animals to viability (Fig. 1B). This same level of Wts expression in wild-type or fat heterozygous animals results in a mild wing vein phenotype and slightly smaller wings, but the flies otherwise appear normal (Fig. 1E and data not shown).
Fig. 1.
Rescue of fat and ex by Wts overexpression. Null mutations in fat or ex are lethal but can be rescued to viability by overexpression of Wts. (A–C) Adult flies of wild type (A), fat8 UAS-Myc:Wts.2/ftG-rv; tub-Gal4 (B), and exe1 UAS-Myc:Wts.2/exe1; tub-Gal4 (C). (D–G) Adult wings from wild type (D), UAS-Myc:Wts.2/tub-Gal4 (E), fat8 UAS-Myc:Wts.2/ftG-rv; tub-Gal4 (F), and exe1 UAS-Myc:Wts.2/exe1; tub-Gal4 (G). The arrows point to extra vein material. (H–J) Portion of abdomens from wild type (H), fat8 UAS-Myc:Wts.2/ftG-rv; tub-Gal4 (I), and exe1 UAS-Myc:Wts.2/exe1; tub-Gal4 (J). In H and J all hairs and bristles point posteriorly (down); in I bristles and hairs are misoriented and swirling patterns of hairs are visible.
Wts-rescued fat mutants are similar to wild-type animals in terms of their overall size and most aspects of their morphology (Fig. 1B). Their most dramatic phenotype is a planar cell polarity (PCP) phenotype, evident in the misalignment of hairs and bristles. In the abdomen (Fig. 1I), this PCP phenotype was similar to that of unrescued fat mutants (13, 14). However, in the wing (SI Fig. 6), the PCP phenotype is weaker than unrescued fat mutants and instead appears similar to the PCP phenotype of fat mutants that have been partially rescued by expression of the fat intracellular domain (15). The persistence of a PCP phenotype is consistent with studies that have implied the existence of two distinct branches of Fat signaling, one affecting transcription and one dedicated to PCP (14, 16). At the same time, it appears, both from these Warts rescue experiments and from prior studies (14, 15), that at least in the wing a transcriptional branch of Fat signaling contributes to the PCP phenotype of fat mutants.
Wts-rescued fat mutants also exhibit abnormal legs, smaller wings, and wing vein phenotypes (Fig. 1 B and F). When the cross veins are visible, the spacing between them is reduced (Fig. 1F), which is a classic Fat pathway phenotype. It is not yet clear whether these phenotypes result from imperfect rescue of Wts-dependent Fat signaling or a contribution of Wts-independent Fat signaling to wing patterning. Nonetheless, the relatively normal growth and development of these animals confirms that critical functions of Fat are mediated by its regulation of Wts.
Ex appears to affect Wts through Hippo signaling (6) and hence is expected to regulate Wts phosphorylation and/or its association with Mats. If the mechanisms that promote Wts activation were completely eliminated in ex mutants, then overexpressing Wts would not be expected to have any effect. However, if Wts activation were reduced but not abolished in ex mutants, then it is conceivable that elevation of Wts might at least partially rescue ex mutants, because by the law of mass action one could expect increased Wts to result in increased production of active Wts. Indeed, ex-null mutant animals could be partially rescued by overexpression of Wts, resulting in the recovery of adult flies (Fig. 1C). The rescue is incomplete, in that the wing discs and adult wings of these animals are enlarged (Fig. 1G), but, aside from this, and mild wing vein phenotypes, these Wts-rescued ex mutants appear remarkably normal (Fig. 1C). They differ most obviously from Wts-rescued fat mutants in their enlarged wings and in the absence of any visible PCP phenotype (Fig. 1J and SI Fig. 6), which suggests that previously reported influences of ex on PCP (17) reflect the influence of Ex on Wts-dependent transcription.
fat and ex Act in Parallel.
Previously, we reported that Fat affects Wts protein levels and proposed this as a basis for Fat signaling (7). However, others reported an effect of Fat on Ex levels or membrane localization and proposed this as a basis for Fat signaling (4, 5, 8). Analysis of double mutant animals provides a test of whether fat and ex act in a single linear pathway or in parallel pathways, because, if Fat signaled solely through Ex, then ex fat double mutants would be expected to be identical to fat or ex single mutants. Indeed, a critical piece of evidence in favor of the hypothesis that Fat signals through Ex was the claim that ex mutants, fat mutants, and ex fat double mutants have identical phenotypes (4, 5, 8). However, this claim was based on analysis of a single phenotype, the number of interommatidial cells in pupal eyes, which is normally reduced by Hippo pathway-dependent apoptosis (2). One report did acknowledge that ex fat double mutants have stronger overgrowth phenotypes in the head (4), but this phenotype was not well characterized, and its significance was discounted.
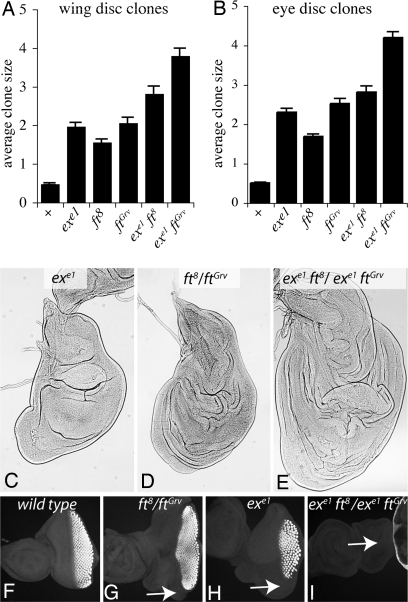
In one assay, the relative areas of mutant clones were measured. For these experiments we used a null allele of ex, exe1, and two different alleles of fat, fat8 and fatGrv. Both of these encode proteins that are truncated in the extracellular domains (15) and lack detectable expression using antibodies directed against the intracellular domain (data not shown). Clones of cells mutant for fat or ex are larger than control clones in both wing and eye discs (Fig. 2 A and B) (14, 18). ex fat double mutant clones were larger than single mutant clones (Fig. 2 A and B). This indicates that fat and ex have additive effects on growth, consistent with the hypothesis that they act in parallel pathways. Unexpectedly, fatGrv clones were larger than fat8 clones. The reason for this difference is not clear, but, because there was a correspondingly greater enhancement of growth in both ex fat double mutant combinations, it does not affect the conclusion that fat and ex have additive effects. As an alternative growth assay, we examined the relative size of the entire wing disk in animals transheterozygous for ex fatGrv and ex fat8 and compared it to the respective single mutants. This confirmed that fat and ex have additive effects, because double mutants have larger discs (Fig. 2 C–E). Many Drosophila tumor suppressors delay pupariation, and the additive influence of fat and ex mutants on disk growth was especially pronounced during this extended larval period. The additive effects of ex and fat mutants on growth confirm that at least some Fat signaling occurs independent of ex.
Fig. 2.
fat and ex have additive effects on growth. (A and B) The average sizes (in arbitrary units) of GFP-expressing clones of the indicated genotypes were measured. Error bars show SEM. For wild-type (+), n (number of clones measured) = 166 for wings and 95 for eyes; for exe1 n = 132 for wings and 154 for eyes; for ft8 n = 114 for wings and 152 for eyes; for ftG-rv n = 133 for wings and 133 for eyes; for exe1 ft8 n = 46 for wings and 70 for eyes; for exe1 ftG-rv n = 184 for wings and 169 for eyes. The increased size of ex fat double mutant clones compared with single mutant clones is significant in all cases (P < 0.01). (C–E) Representative wing imaginal discs of the indicated genotypes are shown from larvae dissected 7 days after egg laying. (F–I) Eye imaginal discs of the indicated genotypes from wandering third-instar larvae stained for ELAV. Arrows point to posterior regions of the eye disk that lack ELAV staining in mutants. All discs are shown at the same magnification.
Although mutation of ex results in overgrowth, the developing eye is actually reduced in size (19). This reduction in the eye field is visible in wandering third-instar larvae stained with a pan-neural antibody, anti-ELAV (Fig. 2H). fat mutants appear to have a modest loss of eye development (Fig. 2G). fat ex double mutants have a strong additive phenotype, because in most cases (28 of 30 discs) they completely failed to initiate eye development, as monitored by ELAV expression (Fig. 2I). Thus fat and ex mutations are additive not only for growth, but also for other phenotypes.
Influence of Fat Signaling on Ex Localization.
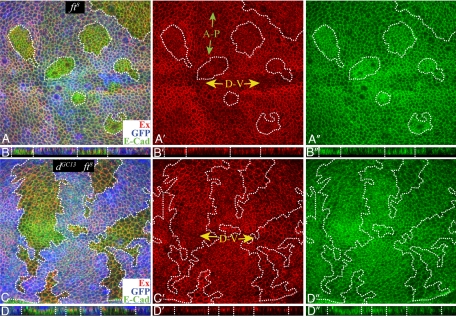
Central to the hypothesis that Fat signals through Ex was the observation that mutation of fat causes a decrease in the levels of Ex at the apical membrane. There are, however, discrepancies among prior reports in terms of the strength of this effect, ranging from a modest decrease (5) to virtually complete loss (4, 8). We have assessed the influence of Fat on Ex by carefully examining Ex protein staining in fat mutant clones throughout the third larval instar, examining both fat8 and fatG-rv clones, in both wing and eye imaginal discs, and throughout the apical-to-basal axis of these discs. We do see in most instances some decrease in the levels of Ex membrane staining within fat mutant clones, although in no case is Ex staining completely lost (Fig. 3 A and B). Confirmation of the specificity of Ex staining in these experiments was provided by examining ex mutant clones, in which loss of Ex is readily detectable (data not shown).
Fig. 3.
Influence of Fat signaling on Ex. Shown are wing imaginal discs stained for Ex (red) and E-cadherin (green). (A and C) Projections through horizontal sections. (B and D) Vertical sections. A′–D′ and A″–D″ show individual channels of the image. Mutant clones (outlined by dashes) were marked by the absence of GFP (blue). GFP staining does not overlap Ex and E-cadherin and is shown from more basal focal planes in the horizontal images. Endogenous Ex staining is lower along the dorsal–ventral boundary (D-V, yellow arrows) and anterior–posterior boundary (A-P, green arrows). (A and B) fat8 mutant clones. Ex staining is decreased. E-cadherin staining sometimes appears increased (20). (C and D) dGC13 fat8 mutant clones. No consistent difference in Ex staining between wild-type and mutant tissue was observed.
In addition to reducing Ex levels at the membrane, fat clones are sometimes associated with a shift in distribution of Ex staining to a more basal focal plane, especially at late third instar. In single horizontal sections (SI Fig. 7), this can give the impression of a substantial loss of Ex staining, which might have contributed to prior reports of almost complete loss of Ex staining. However, in vertical sections (Fig. 3B) or more basal horizontal sections (SI Fig. 7) it is clear that Ex staining is shifted basally rather than lost. To visualize Ex staining in different focal planes within a single horizontal image, we used maximum projection, which reveals a modest decrease in Ex staining (Fig. 3A and SI Fig. 7). Although the basal shift could be a consequence of a specific relocalization of Ex in response to loss of Fat, it could also derive from altered cell shape. To investigate this, fat mutant clones were stained for E-cadherin, which normally localizes near Ex in the subapical membrane. E-cadherin levels often appear slightly elevated within fat mutant clones (20). E-cadherin staining was also shifted basally, in what appears to be a consequence of a change in cell shape rather than a specific effect on the subcellular localization of Ex or E-cadherin (Fig. 3 and SI Fig. 7).
Previously, we described a pathway in which signaling downstream of Fat is mediated by its antagonism of the unconventional myosin Dachs (7, 14, 21). We also identified another Drosophila tumor suppressor, discs overgrown (dco) (22), as a kinase that acts genetically upstream of dachs within the Fat pathway (7). To investigate how the influence of Fat on Ex relates to this branch of Fat signaling, we analyzed their influence on Ex. Ex staining appears slightly reduced in dco3 clones (SI Fig. 8). The influence of dco on Ex is weaker than that of fat, but it also has weaker effects on the expression of downstream target genes (7). The influence of Fat on growth, cell affinity, and gene expression depends completely on dachs (14, 21). The reduction in Ex levels observed in fat mutant clones is similarly dachs-dependent, because in fat dachs double mutant clones Ex staining was indistinguishable from that in surrounding wild-type cells (Fig. 3 C and D).
Fat Signaling Can Occur Independent of Effects on Ex Levels.
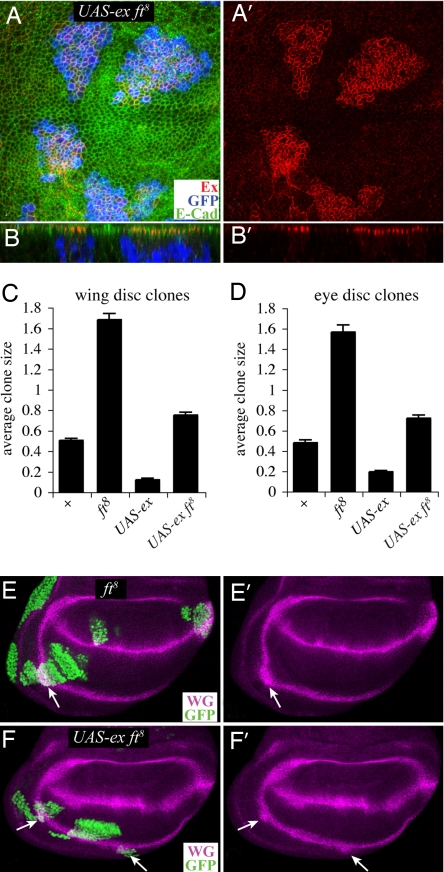
The results described above confirm prior observations that Fat can influence Ex levels at the membrane but do not address the significance of decreased Ex staining to the regulation of growth and gene expression. Thus, to further investigate the relationship between Ex levels and Fat signaling, we examined the influence of fat on Ex overexpression. When the MARCM method (23) was used to overexpress Ex within fat mutant cells, high-level Ex staining was readily detected and appeared at its normal subapical location (Fig. 4 A and B). Thus, although Fat can modulate Ex membrane localization, it is not required for it.
Fig. 4.
Fat signals even when Ex is overexpressed. (A and B) Portions of wing imaginal discs stained for Ex (red) and E-cadherin (green). A shows horizontal sections, and B shows vertical sections. MARCM clones mutant for fat8 and overexpressing Ex were marked by the presence of GFP (blue). Ex staining was captured with intensity settings different from those in Fig. 3 to illustrate the difference in expression levels between endogenous Ex (barely visible) and ectopic Ex (bright red); the localization of ectopic Ex appears normal. (C and D) The average sizes of GFP-expressing clones of the indicated genotypes. Error bars show SEM. For wild-type, n = 166 for wings and 95 for eyes; for ft8 n = 114 for wings and 152 for eyes; for UAS-ex n = 61 for wings and 82 for eyes; for ft8 UAS-ex n = 68 for wings and 102 for eyes. (E and F) Wing imaginal discs stained for WG (magenta) and containing clones (arrows, marked by GFP, green) mutant for fat8 (E) or mutant for fat8 and overexpressing Ex (F).
The observation of elevated Ex staining within these clones provided an opportunity to further explore the relationship between fat and ex. Prior studies have indicated that Ex overexpression appears to mimic Ex gain of function, resulting in a Wts-dependent induction of apoptosis and repression of downstream targets of Hippo signaling (6, 7, 17). Consequently, Ex-expressing clones are greatly reduced in size and number (Fig. 4 C and D) (7, 9). Conversely, fat mutant clones overgrow compared with wild-type clones. Clones of cells mutant for fat and overexpressing Ex exhibited an intermediate phenotype (Fig. 4). These clones are slightly overgrown compared with wild-type control clones and substantially overgrown compared with Ex-expressing clones (Fig. 4 C and D). The observation that mutation of fat dramatically enhances the growth of Ex-expressing clones, even though Ex staining remains strong at the subapical membrane, clearly argues against models in which Fat signals primarily through modulation of Ex levels or localization. By contrast, it is consistent with the hypothesis that fat and ex act in parallel to regulate growth. As a further test, we examined the expression of downstream target of Wts and Yorkie activity. Wg expression in the proximal wing is up-regulated by mutation of fat or by mutation of any of the tumor suppressors in the Hippo pathway (Fig. 4E) (7, 21). Conversely, overexpression of Ex represses Wg expression in the proximal wing (7). Clones of cells mutant for fat and overexpressing Ex exhibit an elevation of Wg expression (Fig. 4F), further demonstrating that Fat signaling can occur independent of an effect on Ex levels or localization.
Discussion
Fat and Ex Act Through Wts.
The observation that fat and ex mutants are rescued to viability simply by overexpressing Wts (Fig. 1) provides a powerful argument that regulation of Wts is their most critical function and against the hypothesis that the influence of Fat and Ex on growth stems from combinatorial effects on many pathways, due to influences on endocytosis or membrane composition (9, 12). Instead, reported effects of ex on other pathways or processes are likely mediated downstream of Wts. Indeed, whereas an increase in Fat protein staining in Mer ex double mutants was interpreted as supporting a general influence of Mer and Ex on endocytosis (12), we have observed a similar effect on Fat staining in clones of cells mutant for wts or overexpressing Yorkie (C. Rauskolb and K.I., unpublished observations), which implies that the influence of Mer and ex on Fat is actually mediated through their influence on transcription. Although the incomplete rescue of ex mutants might be taken as evidence that Ex regulates growth in part independent of Wts, it is also possible that it is simply not possible to generate wild-type levels of active Wts in ex mutants by overexpression.
Parallel Action of Fat and Ex.
The nature of the processes that regulate the Hippo pathway has been a major question in the field—hence the critical importance of the claim that Fat acts as the Hippo receptor. This claim was based largely on three observations. First, it was reported that mutation of fat leads to a substantial loss of Ex protein from its normal subapical location. In fact, however, Ex levels are only partially reduced (Fig. 3 and SI Fig. 7). Moreover, even when Ex levels are artificially elevated, Fat has strong effects, because mutation of fat completely reverses the consequences of Ex overexpression and even leads to some elevation of growth and downstream gene expression (Fig. 4). This indicates that Fat can regulate Wts independent of any effects on Ex levels or localization. This result contradicts that of Tyler and Baker (9), but we confirmed both the overexpression of Ex (Fig. 4) and the absence of Fat (data not shown) by antibody staining and quantified the effects of >400 clones in two different discs using two different fat alleles. Others have claimed that mosaic loss of fat had no effect on Ex overexpression phenotypes during late eye development (4, 8), but close examination of their figures suggests that an intermediate phenotype is actually observed, which would be consistent with our results.
A second argument that Fat acts through Ex to regulate Hippo signaling was provided by the claim that ex fat double mutants exhibit the same phenotype as fat or ex single mutants (4, 5, 8). Although this may be the case in terms of their influence on the number of interommatidial cells, it is not true for other fat and ex phenotypes (Fig. 2). Instead, analysis of ex fat double mutants, together with Ex overexpression in fat mutants, confirms that Fat and Ex can act in parallel. We note that our results do not exclude the possibility that Fat can also act through Ex, whether through its effect on Ex levels or an effect on Ex activity, but they do clearly indicate that at least some Fat signaling occurs independent of Ex.
A third argument was a reported influence of Fat on Wts and Hpo phosphorylation (4, 5). However, this effect has been observed only in experiments in which an intracellular fragment of Fat is expressed at high levels within cultured cells. It could not be observed when full-length Fat was expressed, and it has not been observed in vivo. Because fat can affect Ex staining in discs (Fig. 3) (4, 5, 8), it is plausible that high-level expression of the Fat intracellular domain might influence Ex in cultured cells and thereby influence Hippo signaling. However, the relevance of this mechanism to normal Fat and Hippo signaling in vivo remains to be determined. By contrast, an influence of fat on Wts protein levels has been reported in vivo using simple loss-of-function mutations for fat and examining endogenous Wts protein (7). Moreover, the observation that genetically fat can act independent of ex clearly supports the conclusion that effects of Fat that are independent of Ex, such as its influence on Wts levels, are important.
What then is the functional significance of the influence of Fat on Ex? One possibility is that Fat signals both through an effect on Wts levels (independent of Ex) and through an effect on Wts activity (via Ex). A dual pathway mechanism like this could ensure a robust response to Fat signaling. Resolving whether Fat normally signals through Ex will require reagents for monitoring Wts activity in vivo, which do not yet exist. This dual pathway hypothesis also raises the interesting possibility that the respective contributions of these pathways to Fat signaling could vary in different developmental contexts.
An alternative explanation for the effect of Fat on Ex is suggested by the realization that the discernible effect of fat on Ex protein staining underestimates the actual effect, because ex transcription is up-regulated within fat mutant clones (4, 5, 7). Mutations in components of the Hippo pathway (e.g., wts) elevate both ex mRNA levels and Ex protein staining (6). These observations suggest that a negative influence of Fat signaling on Ex protein accumulation might act as a homeostatic mechanism, maintaining low amounts of Ex at the subapical membrane despite increases in ex mRNA. This could, for example, facilitate the continued regulation of Hippo signaling through Ex independent of Fat.
The Fat–Hippo Signaling Network.
Currently we can describe at least three signal transduction processes downstream of Fat: an influence on Wts levels, an influence on Ex levels, and an influence on PCP (SI Fig. 9). The influence of Fat on PCP is separable from its influences on transcription, because overexpression of Wts only partially rescues fat PCP phenotypes (Fig. 1) and mutation of dachs only partially suppresses fat PCP phenotypes (14). However, the persistence of a some PCP phenotype in fat dachs double mutants, together with the polarized distribution of Dachs protein (14), raises the possibility that Dachs, along with Atrophin (16), might have an input into PCP (SI Fig. 9).
Although we have found that the influence of Fat on both Wts and Ex requires Dachs, these processes are distinct because wts mutation does not decrease Ex levels (4–6) and ex does not influence Wts levels (7). Instead, our results imply that two parallel pathways converge on a common target, Wts, but regulate it in distinct ways, with Hippo signaling influencing Wts activity (1, 2) and Fat signaling influencing Wts levels (7) (SI Fig. 9). Although our results show that these pathways can act in parallel, it is clear that they intersect at multiple points, forming what might be better described as a Fat–Hippo signaling network (SI Fig. 9). Points of intersection include regulation of Wts, regulation of Ex, and transcriptional feedback regulation of network components including four-jointed and ex. A fuller understanding of the relationship between these pathways awaits the identification of additional regulatory inputs into Ex and Mer activity and of reagents to monitor Wts and Yorkie activity in vivo.
Materials and Methods
Drosophila Stocks and Crosses.
For examination of Myc:Wts expression, yw; UAS-Myc:Wts.2/CyO and yw; UAS-Myc:Wts.1/TM6b were crossed to ptc-Gal4 UAS-GFP.
Negatively marked clones were generated by crossing yw; ft8 FRT40A/CyO-GFP, yw; ftGrv FRT40A/CyO-GFP, or yw; dGC13 ft8 FRT40A/CyO-GFP to yw hs-FLP; Ubi-GFP FRT40A/CyO or yw hs-FLP;M(2) Ubi-GFP FRT40A/CyO, and yw; dco3 FRT82B/TM6b to yw hs-FLP; Ubi-GFP FRT82B/CyO.
Positively marked (MARCM) clones were generated by crossing w; exe1 FRT40A/CyO-GFP, yw; exe1 ft8 FRT40A/CyO-GFP, yw; exe1 ftGrv FRT40A/CyO-GFP, yw; ft8 FRT40A/CyO-GFP, yw; ftGrv FRT40A/CyO-GFP, yw; UAS-ex/TM6b, or yw; ft8 FRT40A/CyO-GFP; UAS-ex/TM6b to yw hs-FLP tub-Gal4 UAS-GFP; FRT40A tub-Gal80.
For Wts rescue experiments, we crossed fat8 UAS-Myc:Wts.2/CyO to ftG-rv tub-Gal4[LL7]/TM6b, UAS-Myc:Wts.2 to tub-Gal4[LL7]/TM6b, and exe1 UAS-Myc:Wts.2/CyO to exe1/CyO; tub-Gal4[LL7]/TM6b.
Histology and Imaging.
Discs were fixed and stained as described previously (21) using mouse anti-Wg (1:800, 4D4; Developmental Studies Hybridoma Bank), guinea pig anti-Ex (1:5,000, R. Fehon, University of Chicago, Chicago), rat anti-E-cadherin (1:40, DCAD2; Developmental Studies Hybridoma Bank), mouse anti-Myc (9E10, 1:800; Babco), rat anti-ELAV (Developmental Studies Hybridoma Bank), and rat anti-Fat. Fluorescent stains were captured on a Leica TCS SP5. For horizontal sections, maximum projection using Leica software was used to allow visualization of staining in different focal planes. This method takes the brightest pixel at any given xy position in each of a series of z sections being projected.
For adult tissues, combineZM software was used to allow visualization of features in different focal planes within a single image.
Size Measurements.
To measure clone sizes, embryos were collected from corresponding crosses for 12 h. Forty-eight hours later, larvae were heat-shocked at 36°C for 10 min, and an additional 72 h later animals were dissected and fixed. Clone sizes were measured by tracing in NIH Image J. Samples from different genotypes were collected and analyzed in parallel.
To compare disk sizes, embryos were collected from corresponding stocks or crosses for 8 h and animals were dissected and fixed at appropriate stages as mentioned in the text.
Supplementary Material
ACKNOWLEDGMENTS.
We thank R. Fehon, J. Jiang, H. McNeill, Z. C. Lai, M. Mlodzik, K. Harvey, G. Halder, the Developmental Studies Hybridoma Bank, and the Bloomington Stock Center for antibodies and Drosophila stocks and C. Rauskolb for comments on the manuscript. This research was supported by the Howard Hughes Medical Institute and National Institutes of Health Grant GM078620.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706722105/DC1.
References
- 1.Pan D. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 2.Edgar BA. Cell. 2006;124:267–273. doi: 10.1016/j.cell.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Huang J, Wu S, Barrera J, Matthews K, Pan D. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Willecke M, Hamaratoglu F, Kango-Singh M, Udan R, Chen CL, Tao C, Zhang X, Halder G. Curr Biol. 2006;16:2090–2100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Silva E, Tsatskis Y, Gardano L, Tapon N, McNeill H. Curr Biol. 2006;16:2081–2089. doi: 10.1016/j.cub.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 7.Cho E, Feng Y, Rauskolb C, Maitra S, Fehon R, Irvine KD. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 8.Bennett FC, Harvey KF. Curr Biol. 2006;16:2101–2110. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 9.Tyler DM, Baker NE. Dev Biol. 2007;305:187–201. doi: 10.1016/j.ydbio.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClatchey AI, Giovannini M. Genes Dev. 2005;19:2265–2277. doi: 10.1101/gad.1335605. [DOI] [PubMed] [Google Scholar]
- 11.McCartney BM, Kulikauskas RM, LaJeunesse DR, Fehon RG. Development. 2000;127:1315–1324. doi: 10.1242/dev.127.6.1315. [DOI] [PubMed] [Google Scholar]
- 12.Maitra S, Kulikauskas RM, Gavilan H, Fehon RG. Curr Biol. 2006;16:702–709. doi: 10.1016/j.cub.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence PA, Casal J, Struhl G. Development. 2004;131:4651–4664. doi: 10.1242/dev.01351. [DOI] [PubMed] [Google Scholar]
- 14.Mao Y, Rauskolb C, Cho E, Hu WL, Hayter H, Minihan G, Katz FN, Irvine KD. Development. 2006;133:2539–2551. doi: 10.1242/dev.02427. [DOI] [PubMed] [Google Scholar]
- 15.Matakatsu H, Blair SS. Development. 2006;133:2315–2324. doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]
- 16.Fanto M, Clayton L, Meredith J, Hardiman K, Charroux B, Kerridge S, McNeill H. Development. 2003;130:763–774. doi: 10.1242/dev.00304. [DOI] [PubMed] [Google Scholar]
- 17.Blaumueller CM, Mlodzik M. Mech Dev. 2000;92:251–262. doi: 10.1016/s0925-4773(00)00246-x. [DOI] [PubMed] [Google Scholar]
- 18.Boedigheimer MJ, Nguyen KP, Bryant PJ. Dev Genet. 1997;20:103–110. doi: 10.1002/(SICI)1520-6408(1997)20:2<103::AID-DVG3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 19.Pellock BJ, Buff E, White K, Hariharan IK. Dev Biol. 2007;304:102–115. doi: 10.1016/j.ydbio.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaiswal M, Agrawal N, Sinha P. Development. 2006;133:925–935. doi: 10.1242/dev.02243. [DOI] [PubMed] [Google Scholar]
- 21.Cho E, Irvine KD. Development. 2004;131:4489–4500. doi: 10.1242/dev.01315. [DOI] [PubMed] [Google Scholar]
- 22.Zilian O, Frei E, Burke R, Brentrup D, Gutjahr T, Bryant PJ, Noll M. Development. 1999;126:5409–5420. doi: 10.1242/dev.126.23.5409. [DOI] [PubMed] [Google Scholar]
- 23.Lee T, Luo L. Trends Neurosci. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.