Abstract
We identified a mechanism whereby retina regeneration in the embryonic chick can be induced by the contribution of stem/progenitor cells. We show that bone morphogenetic protein (BMP) signaling is sufficient and necessary to induce retina regeneration and that its action can be divided into two phases. By 3 days after postretinectomy (d PR), the BMP pathway directs proliferation and regeneration through the activation of Smad (canonical BMP pathway) and the up-regulation of FGF signaling by the MAPK pathway. By 7d PR, it induces apoptosis by activating p38 (a noncanonical BMP pathway) and down-regulating FGF signaling (by both MAPK and AKT pathways). Apoptosis at this later stage can be prevented, and BMP-induced regeneration can be further induced by inhibition of p38. These results unravel a mechanism for stem/progenitor cell-mediated retina regeneration, where BMP activation establishes a cross-talk with the FGF pathway and selectively activates the canonical and noncanonical BMP pathways. Retina stem/progenitor cells exist in other species, including humans. Thus, our findings provide insights on how retinal stem cells can be activated for possible regenerative therapies.
Keywords: p38, FGF
Bone morphogenetic proteins (BMPs) are secreted signaling proteins that elicit their effect by binding to a heterodimer receptor complex composed of a BMP type I receptor (BMPRIA or BMPRIB) and a BMP type II receptor (BMPRII) (1). The BMP pathway can activate the canonical downstream effector, Smad, or a noncanonical downstream effector, transforming growth factor-β-activated kinase (TAK1) (1). Several endogenous inhibitors, including noggin, chordin, follistatin, and gremlin, can regulate the ability of BMP to activate these pathways (2).
In the developing retina, BMP2, BMP4, and BMP7, as well as the BMP receptors, are expressed in the chick (3) and mouse (4–6) and have been found to play a role in establishing the dorsal/ventral patterning of the retina. They also regulate the differentiation and survival of retinal neurons (7–11). Because of the BMP pathway's importance during retina development and in stem cell biology, we wanted to examine its role in inducing and regulating retina regeneration.
A population of retinal stem cells is maintained after retinal development in the anterior margin of the eye in many vertebrates, including humans (12, 13). In most vertebrates, these retinal stem cells remain quiescent and do not respond to injury. However, cells in the anterior margin of the embryonic chick eye respond to injury during a limited time of retina development, providing an opportunity to study the induction process of these stem/progenitor cells (13–15).
The embryonic chick has been shown to regenerate a complete retina in ≈7 days as long as a retinectomy is performed on or around embryonic day 4 (E4) and a source of FGF is added (14, 16, 17). In the presence of ectopic FGF2, regeneration takes place by transdifferentiation of the retinal pigmented epithelium (RPE) and the activation of retinal stem/progenitor cells (RS/RPCs) present in the anterior region of the eye known as the ciliary margin (CM), which ultimately houses the ciliary body and ciliary marginal zone (14, 16).
Here we report that BMP signaling regulates the FGF pathway and is capable of inducing retina regeneration from RS/RPCs present in the anterior region of the chick eye in the absence of ectopic FGF. Further detailed analysis revealed that the BMP pathway induces proliferation early in regeneration and apoptosis late in regeneration through an intricate cross-talk with the FGF signaling pathway and by selectively turning on the canonical/noncanonical BMP pathways.
Results and Discussion
Molecules from the BMP Pathway Are Expressed in Chick Eyes at E4 and During Retina Regeneration.
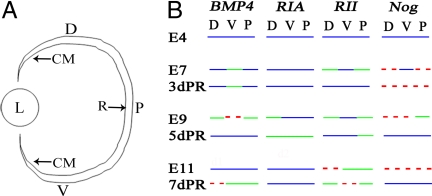
We performed in situ hybridization to determine whether BMP4, BMPRIA, BMPRII, and noggin were expressed at E4, the stage at which retinectomies were performed for the regeneration studies. Because BMP4 is expressed in the dorsal anterior retina at E3, whereas BMP5, BMP6, and BMP7 are only expressed in the developing RPE or optic stalk (3), we concentrated on the expression of BMP4 at E4. BMP4 was expressed at E4 in the dorsal anterior retina, with expression emerging in the posterior and ventral anterior retina as well [Fig. 1 A and B and supporting information (SI) Fig. 7]. BMPRIA, BMPRII, and noggin also were expressed uniformly throughout the retina at E4 (SI Fig. 7).
Fig. 1.
Expression of BMP molecules during retina development and regeneration. (A) Schematic diagram of the eye showing the dorsal (D), ventral (V), and posterior (P) retina and the location of the ciliary margin (CM). L, lens; R, retina. (B) Summary comparing the expression of BMP4, BMPRIA (RIA), BMPRII (RII), and noggin (Nog) through development and regeneration. Lines represent relative expression levels in the dorsal, ventral, and posterior retina from left to right. Red dashes represent no expression, green lines represent relatively low expression, and blue lines represent relatively higher expression.
To determine how the expression pattern of these BMP molecules changes in response to retinectomy and induction of regeneration, we removed the retina at E4 and induced regeneration with FGF2. We collected embryos and performed in situ hybridization at 3, 5, and 7 days postretinectomy (d PR). A summary comparing the expression of the BMP molecules through regeneration and equivalent developmental stages is shown in Fig. 1. The in situ hybridization results during development and regeneration are shown in SI Figs. 7 and 8. Members of the BMP pathway are endogenously expressed during development at the time of retinectomy (E4), and their expression pattern changes from their normal developmental pattern during the regeneration process (compare E7, E9, and E11 to 3d PR, 5d PR, and 7d PR, respectively). The observation that noggin is expressed at E4, but is down-regulated through the initial stages of regeneration (3d PR), suggested that endogenous BMP activity was necessary for the regeneration induced by FGF2.
BMP Signaling Is Necessary to Induce Retinal Stem/Progenitor Cells.
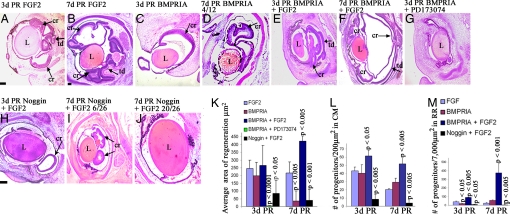
To determine the effect of manipulating the BMP pathway during regeneration, we performed retinectomies at E4 and injected RCAS BMPRIA (a constitutively active form of this receptor) or RCAS noggin in the presence or absence of FGF2. The efficiency of the viral infections is shown in SI Fig. 9. The amount of regeneration in each treated eye was compared with eyes treated with FGF2 alone (Fig. 2 A and B). Analysis at 3d PR revealed that RCAS BMPRIA was able to induce retina regeneration in the absence or presence of ectopic FGF2 (Fig. 2 C and E). The area of regeneration was quantified by using ImagePro software, confirming that there was no significant difference in the amount of regeneration seen in eyes treated with FGF2 (n = 8), RCAS BMPRIA (n = 8), or RCAS BMPRIA + FGF2 (n 18) at 3d PR (Fig. 2K). However, treatment with RCAS BMPRIA + PD173074, an inhibitor of the FGF pathway, resulted in no regeneration at 3d PR, indicating that a functional FGF pathway is necessary for BMP-induced regeneration (P < 0.0001) (n = 4) (Fig. 2 G and K). Inhibition of the endogenous BMP pathway with RCAS noggin resulted in a significant decrease in regeneration even in the presence of ectopic FGF2 at 3d PR (P < 0.05) (n = 11) (Fig. 2 H and K), demonstrating that the endogenous BMP pathway is necessary for the induction of regeneration by FGF2. No regeneration was seen in eyes treated with RCAS noggin alone or with RCAS GFP (data not shown).
Fig. 2.
BMP is sufficient to induce retina regeneration. (A–J) Histological sections stained with H&E at 3d PR (A, C, E, G, and H) or 7d PR (B, D, F, I, and J) in the presence of FGF2 (A and B), RCAS BMPRIA (C and D), RCAS BMPRIA + FGF2 (E and F), RCAS BMPRIA + PD173074 (G), or RCAS noggin + FGF2 (H–J). (D) Representative of 4/12 eyes. (I and J) Representative of 6/26 and 20/26 eyes, respectively. cr, ciliary margin regeneration; td, transdifferentiation; L, lens. (Scale bars: 200 μm; scale bar in B is for C–G and I; scale bar in H is for J.) (K) Quantitative analysis showing the differences in regeneration observed in histological sections. (L and M) Graphical representation of the average number of retinal progenitor cells in the CM region at 3d PR and 7d PR (L) and in the RR (M). P values shown represent significance, compared with eyes treated with FGF2 at each stage. RR, regenerating retina; CM, ciliary margin. The key shown in M also applies to L.
At 7d PR, eyes treated with FGF2 regenerated retina from RS/RPCs present in both the dorsal and ventral CM, resulting in two loops of regenerated retina (n = 10) (Fig. 2B). However, eyes treated with RCAS BMPRIA only had a small loop of regenerated retina at this stage (Fig. 2D) in 33% of chick embryos analyzed and had no regenerated retina in the remaining 67% (n = 12) (data not shown), in strong contrast to what was observed at 3d PR. This treatment resulted in a significant decrease in the area of regeneration, compared with eyes treated with FGF2 at 7d PR (P < 0.005) (Fig. 2K). Despite the decrease in regeneration, immunohistochemical analysis revealed that all retinal cell types were formed in these eyes, as occurs in eyes treated with FGF2 (SI Fig. 10).
However, infection with RCAS BMPRIA in the presence of FGF2 resulted in a significant increase in retina regeneration from the RS/RPCs at 7d PR, compared with eyes treated with FGF2 alone (P < 0.005) (n = 9) (Fig. 2 F and K). A large retinal loop originating from the CM and extra retinal loops at the anterior region were present. Occasionally, the two loops of regenerated retina meet and form one loop in FGF2-treated eyes, but extra retinal loops have not been observed in the anterior region under such conditions. All retinal cell types also formed in these eyes, with a significant increase in the number of ganglion cells and the cells of the inner nuclear layer, compared with the number formed in eyes treated with FGF2 only (SI Fig. 10).
Inhibiting the BMP pathway with RCAS noggin significantly reduced retina regeneration from RS/RPCs in the CM at 7d PR even in the presence of ectopic FGF2 (P < 0.001) (Fig. 2K). In 23% of the eyes treated with RCAS noggin + FGF2, there were small loops of regenerated retina (Fig. 2I) and no evidence of retina regeneration in the remaining 77% (n = 26) (Fig. 2J). Only photoreceptors and Müller glia formed in the treated eyes that had some regeneration, demonstrating the importance of the BMP pathway in differentiation (SI Fig. 10). BMPs have been shown to play a role in the differentiation of the retina during development (9, 11). Thus, a role for the BMP pathway in retinal cell differentiation during retina regeneration is not surprising.
In summary, this histological analysis shows that the BMP and FGF pathways are both necessary for retina regeneration to take place. Ectopic activation of either pathway induced RS/RPCs to regenerate retina only if the other pathway was functional. However, the maintenance of the regenerated retina appears to depend on the presence of FGF2 because the retina is not maintained through 7d PR in eyes treated with RCAS BMPRIA. Therefore, we designed experiments to determine the role of the BMP pathway in the induction and maintenance of regeneration.
BMP Regulates Proliferation, Apoptosis, and Progenitor Cell Number in a Stage-Specific Manner.
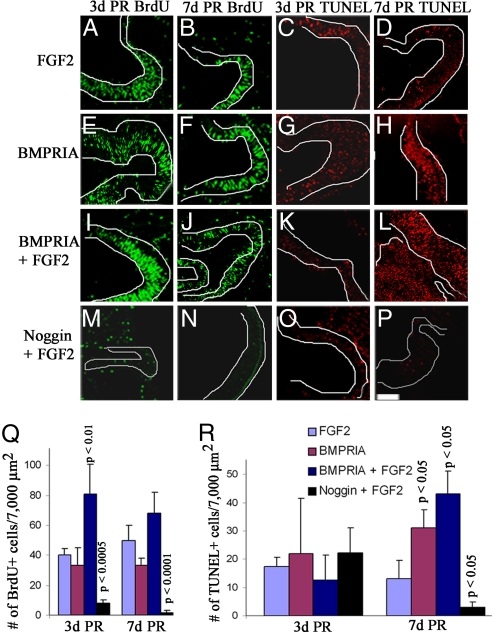
The difference in retina regeneration and the resulting differentiation from the RS/RPCs at 7d PR in each set of treated eyes described earlier could be explained by a change in proliferation, cell survival, and/or the regulation of the retinal progenitor cells. To determine whether BMP played a role in the proliferation of retinal progenitor cells, we analyzed eyes undergoing retina regeneration that received one of the previously mentioned treatments along with BrdU 1 h before collection to identify the number of cells that had entered the S phase during this time period. TUNEL analysis was performed to determine the level of cell death. BrdU+ cells or TUNEL+ cells were counted in six random sections from three different eyes in the anterior region of the regenerating retina, which included the CM as well as ≈ 7,000 μm2 of the regenerated retina (RR).
Retinal progenitor cells express both Pax-6 and Chx-10 (18). Therefore, we performed double-labeling immunohistochemistry and counted the number of retinal progenitor cells in the CM separately from the number of progenitors in the RR in each set of the treated eyes. (We arbitrarily defined the CM as the most anterior 200 μm2.)
Retinal progenitor cells were present in all treated eyes at 3d PR, but the number of progenitors and/or their distribution changed with each treatment (Fig. 2 L and M and SI Fig. 10 Q–T). In eyes treated with FGF2, retinal progenitor cells were present throughout the CM and RR (Fig. 2 L and M). Proliferating cells also were present in the anterior margin of these eyes, but few apoptotic cells were observed (Fig. 3 A and C). The number of progenitors in the CM in eyes treated with RCAS BMPRIA was not significantly different from that in the CM of FGF2-treated eyes (Fig. 2L), but there were significantly less progenitors in the RR in these treated eyes, compared with eyes treated with FGF2 alone (P < 0.05) (Fig. 2M). Also, the number of proliferating or apoptotic cells was not different from the number found in eyes treated with FGF2 (Fig. 3 E, G, Q, and R). This finding suggests that it is possible that BMP signaling increases the rate of differentiation of the retinal progenitors because the number of progenitors is decreased in the RR, but not in the CM, and proliferation and apoptosis are not affected.
Fig. 3.
Activation of BMPRIA induces proliferation during early stages of retina regeneration and apoptosis during late stages of regeneration. (A–P) Retinectomies were performed on E4 chick eyes and collected at 3d PR (A, C, E, G, I, K, M, and O) or 7d PR (B, D, F, H, J, L, N, and P). Eyes were treated with FGF2 (A–D), RCAS BMPRIA (E–H), RCAS BMPRIA + FGF2 (I–L), or RCAS noggin+ FGF2 (M–P). (A, B, E, F, I, J, M, and N) Immunohistochemistry by using an antibody for BrdU (green) shows BrdU+ cells in the anterior region during retina regeneration at each stage. (C, D, G, H, K, L, O, and P) TUNEL analysis shows cells undergoing apoptosis at each stage. (Scale bar: 200 μm; scale bar in P is for all images.) (Q and R) Graphical representation of the average number of BrdU+ (Q) or TUNEL+ (R) cells at each stage in each treated group. P values represent significance, compared with eyes treated with FGF2 at each stage. The key shown applies to both graphs.
Retinal progenitors were distributed uniformly throughout the CM and RR in eyes treated with RCAS BMPRIA + FGF2 at 3d PR, but with a significant increase in number, compared with eyes treated with FGF2 alone (P < 0.05 for CM; P < 0.005 for RR) (Fig. 2 L and M). There also was a corresponding increase in the number of proliferating cells, compared with eyes treated with FGF2 (P < 0.01) (Fig. 3 I and Q), but no change in the level of cell death (Fig. 3 K and R). In contrast, there were significantly fewer progenitor cells in both the CM and RR in eyes treated with RCAS noggin + FGF2 at 3d PR (P < 0.005 for CM; P < 0.05 for RR) (Fig. 2 L and M), with significantly less proliferation compared with eyes treated with FGF2 (P < 0.0005) (Fig. 3 M and Q). Despite the decrease in progenitors in these treated eyes, there was not an increase in apoptosis (Fig. 3 O and R).
Therefore, at 3d PR, RCAS BMPRIA induces proliferation of retinal progenitors without affecting the level of cell death. The presence of ectopic FGF2 enhances the effects of RCAS BMPRIA. FGF2 cannot induce proliferation without a functional BMP pathway as shown by the significant decrease in proliferation in eyes treated with RCAS noggin + FGF2, which most likely accounts for the decrease in regeneration seen in these treated eyes.
At 7d PR, retinal progenitor cells were primarily localized to the CM (Fig. 2 L and M and Fig. 10 U–X) when the eyes were treated with either FGF2 or RCAS BMPRIA, and there was no significant difference in the number of retinal progenitor cells maintained or the number of proliferating cells in each of these groups of treated eyes (Figs. 2 L and M and 3 B, F, and Q). Interestingly, there was a significant increase in the number of apoptotic cells in eyes treated with RCAS BMPRIA, compared with eyes treated with FGF2 at this stage (P < 0.05) (Fig. 3 D, H, and R). Treatment with RCAS BMPRIA + FGF2 caused a significant expansion of the retinal progenitor cells, resulting in a significant increase in the number of progenitors present in both the CM and RR (P < 0.005 for the CM; P < 0.001 for the RR) (Fig. 2 L and M and SI Fig. 10W). There was no significant increase in proliferation, compared with eyes treated with FGF2. However, there was a significant increase in apoptosis, as occurred in eyes treated with RCAS BMPRIA (P < 0.05) (Fig. 3 J, L, Q, and R). Inhibiting the BMP pathway in the presence of FGF2 caused a significant reduction in the maintenance of retinal progenitor cells, as indicated by the loss of Pax-6/Chx-10+ cells in eyes treated with RCAS noggin + FGF2 (P < 0.005 for both the CM and RR) (Fig. 2 L and M and SI Fig. 10X). The cells in the anterior region of the eyes treated with RCAS noggin + FGF2 were not proliferating or undergoing apoptosis (P < 0.0001 for BrdU+ cells; P < 0.05 for TUNEL+ cells, compared with FGF2 treated eyes) (Fig. 3 N and P–R). Therefore, at 7d PR, RCAS BMPRIA induces apoptosis, but it does not increase the level of proliferation above what is seen in eyes treated with FGF2, suggesting that proliferation at this stage is regulated by a separate pathway. The presence of ectopic FGF2 does not interfere with the ability of RCAS BMPRIA to induce apoptosis at this stage.
The amount of retina regeneration seen at 7d PR in eyes treated with RCAS BMPRIA + FGF2 would seem to contradict the increase in apoptosis observed in these eyes. We postulate that the increase in regeneration observed is a result of the increase in proliferating progenitors in these eyes at 3d PR. By 7d PR, proliferation in the RR has decreased and apoptosis has increased, suggesting that, although the retina is still present, it is beginning to degenerate.
Alternatively, because there is an increase in the number of cells in eyes treated with RCAS BMPRIA + FGF2, the increase in apoptosis in these eyes also could be because of the retina attempting to regulate the increase in the number of cells induced by the treatment and not directly a result of the presence of constitutive BMP signaling. To test this theory, we injected RCAS BMPRIA into developing eyes from E6–E11 and found there is an increase in apoptosis, although there is not a corresponding increase in cells entering the cell cycle (SI Fig. 11). This result would argue that BMP is directly involved in inducing the apoptotic pathway at later stages. Moreover, BMPs have been shown to induce apoptosis in the developing retina (10, 19), further supporting our results.
BMPRIA Activates Smad at 3d PR and p38 at 7d PR During Retina Regeneration.
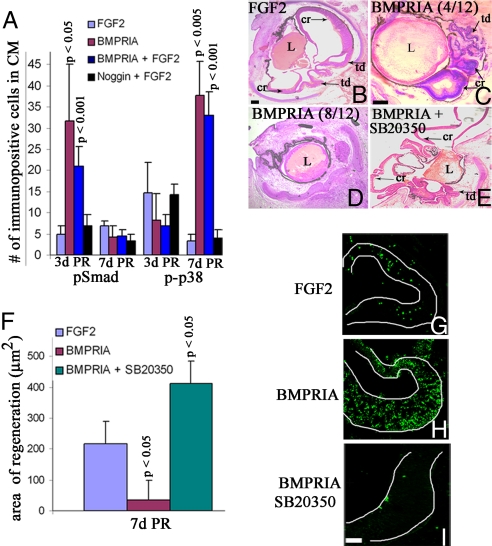
Because RCAS BMPRIA appears to be inducing proliferation during the early stages of regeneration and apoptosis during the late stages of regeneration, we wanted to determine what downstream effectors were activated in each treatment group during each stage of retina regeneration. Smad and p38 can both be activated by BMPRIA as a result of BMP binding (2). Thus, we performed immunohistochemistry on eyes undergoing retina regeneration in the presence of FGF2, RCAS BMPRIA, RCAS BMPRIA + FGF2, and RCAS Noggin + FGF2 to determine the presence of phospho-Smad (pSmad) and phospho-p38 (p-p38) at 3d PR and 7d PR in the CM.
In the presence of FGF2 or RCAS Noggin + FGF2, pSmad was only present in a few cells at 3d PR and 7d PR (5 ± 2 cells for FGF2 at 3d PR; 7 ± 3 cells for RCAS Noggin + FGF2 at 3d PR; 7 ± 1 cells for FGF2 at 7d PR; 3 ± 2 cells for RCAS Noggin + FGF2 at 7d PR) (Fig. 4A). However there was a significant increase in the number of pSmad+ cells in the presence of RCAS BMPRIA, but only at 3d PR (Fig. 4A). In eyes treated with RCAS BMPRIA, there were 32 ± 13 immunopositive cells (P < 0.05, compared with eyes treated with FGF2) at 3d PR, but only 4 ± 3 immunopositive cells at 7d PR. Likewise, eyes treated with RCAS BMPRIA + FGF2 showed 21 ± 5 immunopositive cells (P < 0.001, compared with eyes treated with FGF2 only) at 3d PR, but only 5 ± 2 immunopositive cells at 7d PR.
Fig. 4.
Activation of BMPRIA induces pSmad at 3d PR and p-p38 at 7d PR. Inhibition of p-p38 maintains regenerated retina and inhibits apoptosis. (A) Graphical representation of the average number of pSmad and p-p38-immunopositive cells at 3d PR and 7d PR in the CM during retina regeneration. P values represent significance, compared with eyes treated with FGF2 at each stage. (B–E) Histological sections at 7d PR of eyes treated with FGF2 (B), RCAS BMPRIA in 4 of 12 cases (C) and 8 of 12 cases (D), or RCAS BMPRIA + SB20350 (E). L, lens; cr, ciliary margin regeneration; td, transdifferentiated retina. (Scale bars: 100 μm; scale bar in B is also for D and E.) (F) Quantification of the regenerated area in all treated eyes. P values represent significance, compared with eyes treated with FGF2 only. (G–I) TUNEL analysis of eye treated with FGF2 (G), RCAS BMPRIA (H), and RCAS BMPRIA + SB20350 (I). (Scale bar: 200 μm; scale bar in I is also for G and H.)
There was a relatively low number of p-p38+ cells in all treated eyes at 3d PR in the CM (15 ± 3 cells for FGF2-treated eyes; 8 ± 7 cells for eyes treated with RCAS BMPRIA; 7 ± 6 cells for RCAS BMPRIA + FGF2; 14 ± 3 cells for RCAS noggin + FGF2) (Fig. 4A). At 7d PR, however, there was a significant increase in p-p38-immunopositive cells in the CM of eyes treated with RCAS BMPRIA (38 ± 8; P < 0.005) and RCAS BMPRIA + FGF2 (33 ± 6; P < 0.001), compared with eyes treated with FGF2 (3 ± 2) (Fig. 4A).
The downstream effector of the BMP pathway activated by RCAS BMPRIA depended on the stage of regeneration. RCAS BMPRIA activated Smad during the early stages of regeneration and activated p38 during the late stages of regeneration, presumably inducing the proliferation and apoptosis observed, respectively, during these stages. We also found that RCAS BMPRIA activated Smad and induced cell-cycle entry when injected into developing eyes from E2.5–E4 and activated p38 and induced apoptosis when injected between E6–E11, corroborating the context-dependent activation of Smad and p38 (SI Fig. 11).
It has been postulated that BMPs regulate the distinct processes of proliferation and apoptosis in a context-dependent manner during retina development as well. For example, during chick eye development, inhibition of the BMP pathway at the optic vesicle stage by overexpression of the BMP antagonist, gremlin, showed an increase in apoptosis (8), whereas inhibition of the BMP pathway later in eye development, during optic cup formation, showed a decrease in apoptosis (10). Likewise, a conditional mutant mouse lacking both copies of BMPRIA and BMPRIB showed that proliferation in the retina is unaffected by the decrease in BMP activity early in development, but decreases proliferation later in development (9), indicating the context is important for the observed effects.
Inhibition of p-p38 Activity Allows Maintenance of RCAS BMPRIA-Induced Regeneration.
Because activation of p38 by BMP is associated with an increase in apoptosis (20, 21), we hypothesized that the activation of p38 by RCAS BMPRIA resulted in an increase in apoptosis, and this result was the reason the regenerated retina was not maintained beyond 3d PR in these eyes. To test this hypothesis, we performed retinectomies at E4 and added RCAS BMPRIA + SB20350, an inhibitor that prevents p-p38 from activating its downstream targets. We collected the eyes 7d PR and processed them for histology and immunohistochemistry.
In contrast to the reduction in regeneration seen at 7d PR in eyes treated with RCAS BMPRIA, compared with eyes treated with FGF2 (Fig. 2 B and D), eyes treated with RCAS BMPRIA + SB20350 (n = 6) showed a significant increase in regeneration over what is observed in eyes treated with RCAS BMPRIA or FGF2 (P < 0.05 for the increase in regeneration with RCAS BMPRIA + SB20350 and for the decrease in regeneration with RCAS BMPRIA alone, compared with FGF2) (Fig. 4 B–F). TUNEL analysis also showed that there was no apoptosis occurring in eyes treated with RCAS BMPRIA + SB20350, compared with the low level of apoptosis in eyes treated with FGF2 and the high level of apoptosis in eyes treated with RCAS BMPRIA (Fig. 4 G–I). This finding supported our hypothesis that the activation of p38 by RCAS BMPRIA caused an increase in apoptosis and subsequent loss of the regenerated retina.
BMP Regulates the FGF Pathway.
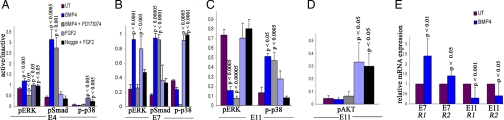
Our results have shown that BMP and FGF were both necessary for retina regeneration. We wanted to determine whether there was cross-talk between these two pathways during the induction and process of retina regeneration especially because ectopic FGF2 enhanced the effect of RCAS BMPRIA at 3d PR. To determine this notion, we isolated the CM containing RS/RPCs from the anterior region of developing chick eyes at E4, E7, and E11 corresponding to the time of rectinectomy, 3d PR, and 7d PR respectively. We incubated the CM explants with BMP4, BMP4 + PD173074, FGF2, or Noggin + FGF2. We then determined the level of phosphorylated ERK (pERK) (a downstream effector of FGF signaling) (22) as well as pSMAD and p-p38 by Western blot analysis for each treatment group. ImageQuant software was used to determine the ratio of the active to inactive protein to show the level of activation for each treatment. The Western blots used for quantitation are shown in SI Fig. 12.
BMP activated Smad at E4 and E7 and p38 at E11 (Fig. 5A–C), agreeing with the results shown in Fig. 4A. In addition to activation of Smad at E4 and E7, BMP also activated ERK, but this activation depended on a functional FGF pathway as shown by the lack of ERK activation when BMP and PD173074 were added together (Fig. 5 A and B). However, at E11, BMP decreased the level of pERK while activating p38 independent of the FGF pathway (Fig. 5C). p38 also can be activated through the FGF pathway (22). In our retina model, FGF2 alone activates p38 at E4 and E7 (Fig. 5 A and B), whereas BMP4 activates p38 at E11 (Fig. 5C). These results suggest that BMP-induced apoptosis depends on p38 and the down-regulation of the FGF pathway, which is known to elicit survival signals especially through the activation of AKT (22). We examined the level of pAKT in E11-treated explants. In these ciliary explants, incubation with BMP4 or BMP4 + PD173074 resulted in no activation of AKT, whereas incubation with FGF2 or noggin + FGF2 resulted in a significant increase in AKT activation (Fig. 5D). Therefore, the activation of AKT in eyes treated with FGF2 could account for the low level of apoptosis observed in these eyes. However, the low level of pAKT in untreated E11 CM explants suggest other mechanisms work with BMP to regulate this arm of the FGF pathway. This finding is not surprising because apoptosis normally occurs during this stage of retina development (23).
Fig. 5.
BMP regulates the FGF pathway. (A–C) Graphical representation of the densitometry measurements showing the ratio of pERK/ERK, pSmad/Smad, and p-p38/p38 at E4 (A), E7 (B), and E11(C). P values represent significance in the ratio of densitometry for each treatment, compared with the untreated control (UT) at each stage. (D) Graphical representation of densitometry measurements showing the ratio of pAKT/AKT in E11-treated explants. The key in A also applies to B, C, and D. (E) Graphical representation of relative mRNA expression for FGFR1 and FGFR2 determined by real-time RTPCR in E7- and E11 BMP-treated explants. R1, FGFR1; R2, FGFR2.
These results suggest that the BMP pathway regulates the FGF pathway during retina regeneration from the CM. To further confirm this finding, we performed real-time RT-PCR by using RNA isolated from E7 and E11 BMP4-treated ciliary explants and examined the expression levels of FGFR1 and FGFR2. We found that BMP increased expression of FGFR1 and FGFR2 in E7 explants (P < 0.010 for FGFR1; P < 0.05 for FGFR2) and decreased expression of both FGFR1 and FGFR2 in E11-treated explants (P < 0.001 for FGFR1; P < 0.05 for FGFR2) (Fig. 5E). We concluded that the BMP pathway may regulate the FGF pathway during retina regeneration by controlling the expression levels of FGF receptors.
Conclusion
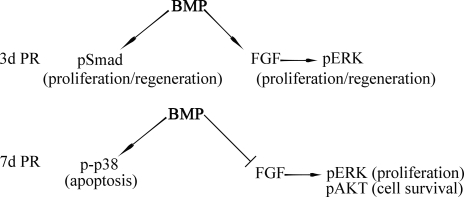
In this study, we found that BMP is sufficient and necessary for the induction of retina regeneration from RS/RPCs present in the CM of the embryonic chick. The BMP pathway directs the process of retina regeneration by differentially activating the canonical (Smad) and noncanonical (p38) pathway, as well as regulating the FGF pathway in a stage-specific manner. Initially, the BMP pathway activates Smad and directs FGF-dependent activation of ERK by increasing the expression of FGFR1 and FGFR2, resulting in proliferation and differentiation. As regeneration proceeds, the BMP pathway down-regulates FGFR1 and FGFR2 and activates p38, instead of Smad regulating apoptosis at this stage. A possible model depicting the role of BMP during the early and late stages of regeneration is represented in Fig. 6.
Fig. 6.
Model depicting the role of BMP in the induction of retina regeneration.
Activation of RS/RPCs in higher vertebrates requires proper regulation of proliferation, differentiation, and apoptosis to adequately regenerate a functional retina. Our studies pinpoint a fundamental role of BMP pathway in these processes. Extension of these studies to include the networking with other pathways will eventually lead to an understanding of the molecular mechanisms that lead to induction of retina regeneration.
Materials and Methods
Surgical Procedure.
White Leghorn chicken eggs were purchased from Ohio State University (Columbus, OH) and incubated in a 38°C rotating incubator. Surgeries were performed as described (14).
In Vivo Treatments.
For proliferation studies, 1 μl of a 10 mM solution of BrdU or 10 μl of a 100 mM solution of BrdU was injected over the eye of the embryo 1 h before collection. Replication-competent RCAS (A) retroviruses engineered to express a constitutively active form of BMPRIA (24) (also described in SI Materials and Methods), GFP, or noggin were produced and injected as described (14). Beads containing FGF2, SB20350 (p38 inhibitor; BioChem), or PD173074 (FGFR inhibitor; Pfizer) were prepared as described (16). See SI Materials and Methods for how eyes were fixed for each procedure and processed for histology, immunohistochemistry, and TUNEL.
Immunohistochemistry.
Details of antibodies and immunohistochemistry are provided in SI Materials and Methods.
In Situ Hybridization.
Probes were prepared from plasmids containing BMP4, BMPRIA, BMPRII, and noggin, and in situ hybridization was performed as described (3).
TUNEL.
TUNEL was performed by using the in situ cell death-detection kit TMR-red (Roche Applied Sciences).
Quantification.
ImagePro Plus software was used for all quantitative analyses. More details are described in SI Materials and Methods. Student's t test was used to determine statistical significance. Error bars represent SEM.
In Vitro Explants.
In vitro explants, Western blot analysis, and real-time RT-PCR was performed as in ref. 16 with the following treatments: 10 μg/ml FGF2 (R&D Systems), 1–2 μg/ml BMP4 (R&D Systems), 60 ng/ml noggin (R&D Systems), and/or 2.5 μM PD173074 (Pfizer). Densitometry measurements were determined by using ImageQuant 5.2 software. Relative mRNA expression levels were determined by using the Pfaffl method (25). Three different biological samples were assayed for each treatment group to confirm results.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Drs. Teri Belecky-Adams (Indiana University–Purdue University, Indianapolis, IN), Lee Niswander (University of Colorado Health Sciences Center, Denver), Cliff Tabin (Harvard Medical School, Boston), and Thomas Jessell (Columbia University, New York) for supplying RCAS viruses, probes, and antibodies; Pfizer for supplying PD173014; Bret Lehman, Amir Abthai, and Svranthi Reddy for their contributions to this work; and Maria Natalia Vergara and Drs. Matthew Grogg, Jason Spence, and Mayur Madhavan for critical review of this manuscript. This work was supported by National Institute of Aging Grant R21 AG 024937-01 (to K.D.R.-T.) and National Eye Institute Grant EY10540 (to P.A.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708202104/DC1.
References
- 1.Nohe A, Keating E, Knaus P, Petersen NO. Cell Signal. 2004;16:291–299. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Balemans W, Van Hul W. Dev Biol. 2002;15:231–250. [PubMed] [Google Scholar]
- 3.Belecky-Adams T, Adler R. J Comp Neurol. 2001;430:562–572. [PubMed] [Google Scholar]
- 4.Dudley AT, Robertson EJ. Dev Dyn. 1997;208:349–362. doi: 10.1002/(SICI)1097-0177(199703)208:3<349::AID-AJA6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 5.Furuta Y, Piston DW, Hogan BL. Development (Cambridge, UK) 1997;124:2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- 6.Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. Dev Biol. 1999;207:176–188. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Wilson S, Reh T. Dev Biol. 2003;256:34–48. doi: 10.1016/s0012-1606(02)00115-x. [DOI] [PubMed] [Google Scholar]
- 8.Huillard E, Laugier D, Marx M. Dev Biol. 2005;283:335–344. doi: 10.1016/j.ydbio.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Murali D, Yoshikawa S, Corrigan RR, Plas DJ, Crair MC, Oliver G, Lyons KM, Mishina Y, Furuta Y. Development (Cambridge, UK) 2005;132:913–923. doi: 10.1242/dev.01673. [DOI] [PubMed] [Google Scholar]
- 10.Trousse F, Esteve P, Bovolenta P. J Neurosci. 2001;21:1292–1301. doi: 10.1523/JNEUROSCI.21-04-01292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adler R, Belecky-Adams TL. Development (Cambridge, UK) 2002;129:3161–3171. doi: 10.1242/dev.129.13.3161. [DOI] [PubMed] [Google Scholar]
- 12.Coles BL, Angenieux B, Inoue T, Del Rio-Tsonis K, Spence JR, McInnes RR, Arsenijevic Y, van der Kooy D. Proc Natl Acad Sci USA. 2004;101:15772–15777. doi: 10.1073/pnas.0401596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haynes T, Del Rio-Tsonis K. Curr Neurovasc Res. 2004;1:231–239. doi: 10.2174/1567202043362216. [DOI] [PubMed] [Google Scholar]
- 14.Spence JR, Madhavan M, Ewing JD, Jones DK, Lehman BM, Del Rio-Tsonis K. Development (Cambridge, UK) 2004;131:4607–6421. doi: 10.1242/dev.01298. [DOI] [PubMed] [Google Scholar]
- 15.Tsonis PA, Del Rio-Tsonis K. Exp Eye Res. 2004;78:161–172. doi: 10.1016/j.exer.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Spence J, Aycinena JC, Del Rio-Tsonis Dev Dyn. 2007;236:1161–1174. doi: 10.1002/dvdy.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park CM, Hollenberg MJ. Dev Biol. 1989;134:201–205. doi: 10.1016/0012-1606(89)90089-4. [DOI] [PubMed] [Google Scholar]
- 18.Belecky-Adams T, Tomarev S, Li HS, Ploder L, McInnes RR, Sundin O, Adler R. Invest Ophthalmol Vis Sci. 1997;38:1293–1303. [PubMed] [Google Scholar]
- 19.Franke AG, Gubbe C, Beier M, Duenker N. J Comp Neurol. 2006;495:263–278. doi: 10.1002/cne.20869. [DOI] [PubMed] [Google Scholar]
- 20.Kendall SE, Battelli C, Irwin S, Mitchell JG, Glackin CA, Verdi JM. Mol Cell Biol. 2005;25:7711–7724. doi: 10.1128/MCB.25.17.7711-7724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura N, Matsuo R, Shibuya H, Nakashima K, Taga T. J Biol Chem. 2000;275:17647–17652. doi: 10.1074/jbc.M908622199. [DOI] [PubMed] [Google Scholar]
- 22.Eswarakumar VP, Lax I, Schlessinger J. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Vecino E, Hernandez M, Garcia M. Int J Dev Biol. 2004;48:965–974. doi: 10.1387/ijdb.041891ev. [DOI] [PubMed] [Google Scholar]
- 24.Zou H, Wieser R, Massague J, Niswander L. Genes Dev. 1997;11:2191–2203. doi: 10.1101/gad.11.17.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaffl MW. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.