Abstract
The size structure of phytoplankton assemblages strongly influences energy transfer through the food web and carbon cycling in the ocean. We determined the macroevolutionary trajectory in the median size of dinoflagellate cysts to compare with the macroevolutionary size change in other plankton groups. We found the median size of the dinoflagellate cysts generally decreases through the Cenozoic. Diatoms exhibit an extremely similar pattern in their median size over time, even though species diversity of the two groups has opposing trends, indicating that the macroevolutionary size change is an active response to selection pressure rather than a passive response to changes in diversity. The changes in the median size of dinoflagellate cysts are highly correlated with both deep ocean temperatures and the thermal gradient between the surface and deep waters, indicating the magnitude and frequency of nutrient availability may have acted as a selective factor in the macroevolution of cell size in the plankton. Our results suggest that climate, because it affects stratification in the ocean, is a universal abiotic driver that has been responsible for macroevolutionary changes in the size structure of marine planktonic communities over the past 65 million years of Earth's history.
Keywords: cell size, climate change, dinoflagellates, evolution, food webs
Marine phytoplankton are a polyphyletic group of unicellular or colonial photoautotrophs (1) that range in size from <1 μm to >1 mm in equivalent spherical diameter, corresponding to >8 orders of magnitude variation in cell volume. Because cell size influences nutrient uptake kinetics, photosynthesis, respiration, growth, and sinking rates (2–5), as well as genome size and rate of evolution (6, 7), the size structure of phytoplankton assemblages strongly influences energy transfer through the food web and tempo of evolution in the sea (8–10). Based on analyses of fossil records over the Cenozoic, macroevolutionary changes in cell size have been reported for marine diatoms (11), coccolithophorids (12), and the amoeboid planktonic foraminifera (13–15); however, the underlying causes of such changes remain unclear. Broadly, hypotheses accounting for macroevolutionary trends fall into two groups: (i) specific biotic or abiotic forcings unique to each taxon or (ii) taxon-specific responses to a universal abiotic factor. Here, based on an analysis of the fossil record, we report that, like the other plankton groups examined, dinoflagellates exhibit an active macroevolutionary change in size in concert with changes in the thermal contrast between the surface and deep ocean. These results strongly suggest that a universal abiotic driver related to climate is responsible for macroevolutionary changes in the size structure of marine planktonic communities over the past 65 million years of Earth's history.
Dinoflagellates are a group of unicellular eukaryotic flagellates characterized by a longitudinal and a transverse flagellum and are often armored with cellulosic plates. There are ≈2,000 extant morphologically identified species that inhabit a wide range of aquatic environments, exhibit a full array of nutritional modes, including photosynthetic, heterotrophic, mixotrophic, parasitic, and symbiotic habits (16); and are often a significant component of modern marine planktonic communities (17). Molecular clock and fossil biochemical markers suggest the dinoflagellates may have been present as far back as the Early Paleozoic (≈500–550 Ma), but it was not until the early Jurassic (≈200 Ma) that they established a consistent and diverse morphologically identifiable fossil record (18–20). Many Dinophyceae, especially the Gonyaulacales and Peridiniales, form cysts with extremely resistant organic cell walls that facilitate their preservation in the fossil record and have made them useful Mesozoic and Cenozoic biostratigraphic markers and paleoenvironmental indicators (21). Using this record of fossil cysts, we examined the macroevolutionary trajectory in the size of this marine planktonic group (photosynthetic and heterotrophic) in relation to recently compiled size records of other planktonic groups.
Results and Discussion
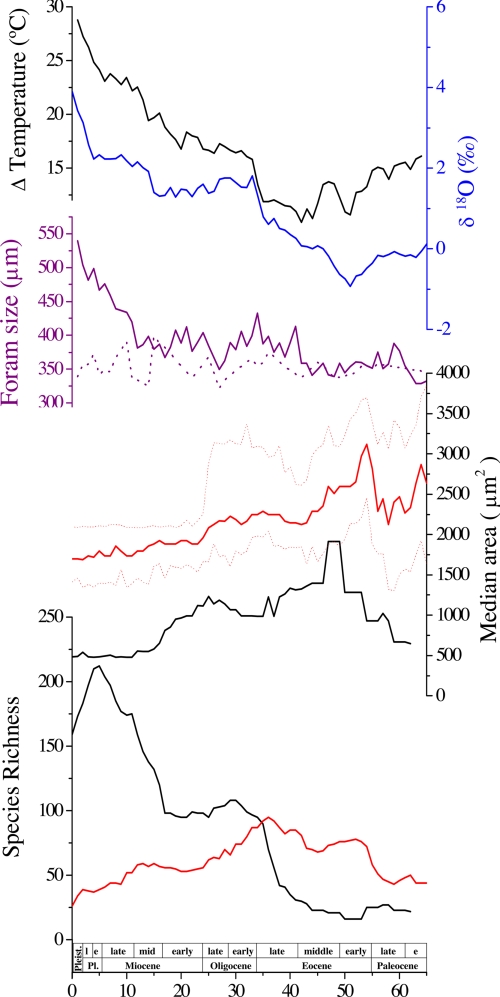
The median size of the dinoflagellate cyst assemblage decreases over the Cenozoic (Fig. 1). Median dinoflagellate cyst size is relatively high immediately after the end-Cretaceous boundary (33% above the Cenozoic average), drops in the Paleocene, and then recovers to reach its maximum just after the Paleocene–Eocene boundary (44% above the average). Through the rest of the Cenozoic, the median size of the dinoflagellate cysts decreases, reaching its minimum in the Pleistocene (22% below the average). The observed trend in the median size of dinoflagellate cysts is in accordance with observed changes in composition of dinoflagellate cyst assemblages in the Cenozoic (21) and their size ranges, as discussed below (Fig. 2).
Fig. 1.
Cenozoic record of species richness of the dinoflagellate cyst (red) and diatom (black) assemblages analyzed in this study, median size (area, μm2) and 95% confidence interval of the dinoflagellate cyst fossil assemblage (red) and median area of diatom assemblage (black) (11) averaged over 3-Ma intervals, planktonic foraminifera size (μm) from the lower (purple, solid) and higher latitudes (purple, dashed, from ref. 13). The deep-sea oxygen isotope δ18O record is in blue, and vertical temperature gradient is in black (44). Note that the diatom and foraminifera size data use the 1995 geological time scale (11, 13).
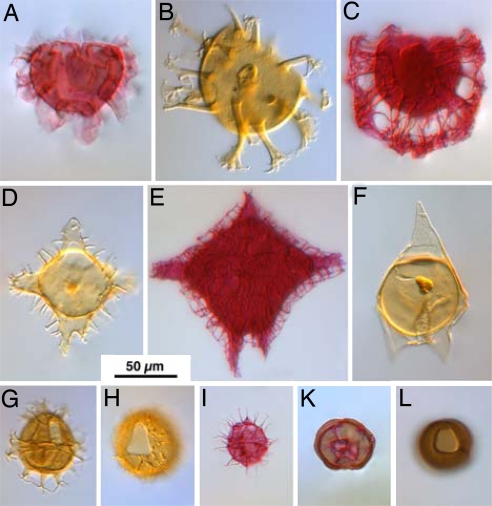
Fig. 2.
Examples of Cenozoic dinoflagellate cyst genera illustrating morphological variety and size range. All specimens to the same scale. Stratigraphic range of the genus in brackets. (A) Areoligera (Campanian to Early Oligocene), (B) Cordosphaeridium (Campanian to Miocene), (C) Glaphyrocysta (Maastrichtian to Oligocene), (D) Wetzeliella (Eocene to Oligocene), (E) Charlesdowniea (Early Eocene to Early Oligocene), (F) Deflandrea (Maastrichtian to Oligocene), (G) Spiniferites (Berriasian to Holocene), (H) Operculodinium (Maastrichtian to Holocene), (I) Phthanoperidinium (Paleocene to Holocene), (K and L) protoperidiniacean cysts; (K) Selenopemphix (Eocene to Holocene), and (L) Brigantedinium (Miocene to Holocene).
Paleocene and Eocene assemblages are rich in large gonyaulacaceans (e.g., the Spiniferites group and large taxa such as Cordosphaeridium), goniodomaceans (e.g., Hystrichosphaeridium and Homotryblium), areoligeraceans (e.g., Areoligera and Glaphyrocysta), and peridiniaceans (e.g., Deflandrea, Wetzeliella, Charlesdowniea, Dracodinium, and Rhombodinium) (Fig. 2 A–G). Dinoflagellate cyst diversity drops dramatically in the Oligocene and continues decreasing throughout the Cenozoic, a trend that has generally been associated with major climatic cooling (e.g., ref. 21). Oligocene assemblages show a marked decline in species of the deflandreacean lineage (sensu ref. 22), with small Phthanoperidinium (Fig. 2I) and large Deflandrea and Wetzeliella and its allies becoming extinct while the protoperidiniaceans (Fig. 2 K–L), which are considerably smaller, continued to thrive and became more diverse and widely distributed. Some Oligocene assemblages are still dominated by large areoligeraceans (e.g., Chiropteridium) and/or moderately sized gonyaulacaceans (e.g., Spiniferites and Operculodinium; Fig. 2 G–H). Neogene dinoflagellate cyst assemblages have a decidedly modern aspect, with small protoperidiniaceans and moderate gonyaulacaceans (Spiniferites and Operculodinium) dominating neritic environments. Small protoperidiniaceans dominate many Miocene to recent higher latitude assemblages.
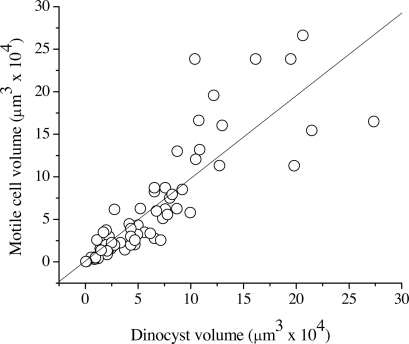
It is estimated that ≈13–16% of living dinoflagellate species have a dormant cyst stage (16). Laboratory investigations of extant dinoflagellates indicate most cysts form from the sexual recombination of two vegetative cells, a process that can be induced by nutrient depletion (23). Resting cysts can play a significant role in the success and persistence of dinoflagellates by allowing the species to survive unfavorable fluctuations in environmental conditions that exceed the physiological tolerances for vegetative growth (24). Indeed, dinoflagellate cysts are able to survive for extended periods and have been shown to hatch successfully after storage for up to 9 years (25). Larger cysts have a higher maximum capacity to store reserve materials, and a lower specific metabolic rate suggesting large cyst size could be an adaptation to extend the maximum temporal viability of the cyst. In addition, larger cyst size increases the sinking rate and so may protect the cyst from pelagic grazers. As a result, macroevolutionary changes in the median size of dinoflagellate cysts over time may reflect a change in the type and frequency of different environmental conditions in the water column and sediment surface with changes in climate. It is difficult to distinguish between the motile phase of the dinoflagellate life cycle and the cyst stage as the unit of selection for the macroevolutionary trajectory in size, because cell size during the motile phase is strongly linearly related to cyst size (Fig. 3).
Fig. 3.
Relationship between cyst size (μm3) and size of cell in motile phase (μm3) gathered from literature data (see SI for a list of references).
Evolutionary trajectories in the size of plankton could be the result of an evolutionary “arms race” between consumers and their prey (26, 27). Many consumers, including common phytoplankton grazers such as copepods, discriminate between prey based on differences in size (28), as well as nutritional quality or toxicity (29–32); thus, size-selective grazing pressure by common consumers could be responsible for the parallel temporal trajectory in the median size of dinoflagellate cyst and diatom frustule assemblages over the Cenozoic. For example, the relatively large median size of dinoflagellate cysts and diatoms in the Paleocene may be a response to selective extinction and slower recovery of the larger zooplankton consumers, resulting in a relative release of grazing pressure on the larger phytoplankton species (33). Further complicating any simple interpretation, it is estimated that ≈50% of extant dinoflagellates may be heterotrophs, some of which feed on diatoms and photosynthetic dinoflagellates, and some species are known to be cannibals (34). The feeding strategies of extant heterotrophic dinoflagellates are quite varied; some are typical phagotrophs, others use a feeding tube to connect to their prey, and some extrude a feeding veil that can envelop and digest prey outside the cell (34). Heterotrophic dinoflagellates prefer prey of similar or slightly larger size than themselves (35, 36). Because the median size of the dinoflagellates is considerably larger than the diatoms, it is unlikely heterotrophic dinoflagellate grazing played an important selective role in the temporal change in the size of the diatoms through the Cenozoic. In addition, the largest change in the size of planktonic foraminiferal tests occurs several million years after a large decrease in the size of the diatoms and dinoflagellate cysts in the Neogene, indicating that foraminferal grazing on dinoflagellates and diatoms is not a primary selective agent in the macroevolutionary trajectory of size in these groups through the Cenozoic (Fig. 1). Unfortunately, the fossil record of most of the major zooplankton groups is weak (37), making it difficult to corroborate or contradict the hypothesis that an evolutionary arms race has driven these macroevolutionary changes in plankton size. Nevertheless, the macroevolutionary trajectory in the size structure of the primary producers will have had a profound influence on the evolution of the size structure of the higher trophic levels (33, 38).
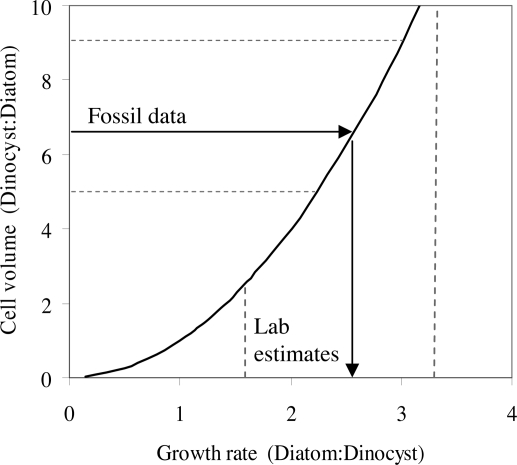
The diatoms and dinoflagellates have the largest cells of the unicellular marine phytoplankton groups. Both groups have a few small species with equivalent spherical diameters near 2 μm and a few large species with linear dimensions that can reach >1 mm, but on average, dinoflagellates are ≈3.4 times larger by area than the diatoms in the youngest fossil assemblage (Fig. 1). An allometric analysis can be used to explore whether nutrient limitation and fundamental differences in growth rates between the dinoflagellates and diatoms can account for the different characteristic sizes of the groups (Fig. 4). Assuming growth rate (μ, h−1) and cell quota (cell nutrient content, Q, mol nutrient cell−1) scale with (V) cell volume [where μ = μkVb1, Q = qkVb2, and μk and qk are μ and Q at V = 1, and b1 and b2 are size-scaling exponents on μ and Q, respectively (39)], and that in situ growth rates are determined by the balance of nutrient supply and demand, μ·Q, then a simple allometric model predicts:
if qk does not significantly differ between taxonomic groups. Typical values of b1 (−0.25) and b2 (0.75) predict diatom growth rates (μkDiatom) must be ≈2- to 3-fold faster than dinoflagellate growth rates (μkDino) to explain the difference in median dinoflagellate cyst and diatom size (39). Laboratory and field data confirm that dinoflagellates do have slower size-normalized growth rates than diatoms (40, 41). The success of the nutrient-constrained allometric model in predicting the ratio of the median size of diatoms and dinoflagellates provides corroborating evidence that nutrient availability has acted to select phytoplankton of different sizes over the Cenozoic (Fig. 4).
Fig. 4.
Model prediction of the evolutionary dinoflagellate-to-diatom cell size ratio (cell volume, V), due to the ratio of their size-normalized growth rates (μk) under nutrient-limited conditions (solid black line). The arrow on the y axis marks the median volume ratio of dinoflagellates:diatoms from the fossil assemblage at 0 Ma, with the 95% confidence interval (dashed lines on the y axis) based on the median dinoflagellate cyst size. The arrow on the x axis marks the size-normalized ratio of diatom:dinoflagellate growth rate predicted by the model using the fossil data. The dashed lines on the x axis indicate the range of size-normalized diatom:dinoflagellate growth rates from laboratory experiments (41, 60).
The shifts in the median size of the dinoflagellate cysts are highly correlated with Cenozoic climate. The maxima in the median size of the dinoflagellate cyst assemblage at ≈54 Ma occurs during the warmest greenhouse climatic conditions of the Cenozoic, namely the early Eocene climatic optimum (42). Decreases in the median size of cysts from early to middle Eocene correspond to an initial secular cooling trend in the Eocene. Similarly, the marked Miocene decreases in the median size of the dinoflagellate cysts correspond with the major paleoceanographic reorganization that began in the earliest Miocene with the major Mi-1 glaciation and continued through mid-Miocene with the intensification of polar cooling, expansion of Antarctic ice sheets, and growth of northern hemisphere ice sheets (42). The Miocene glaciation appears to have been caused by a coordinated combination of a minimum in eccentricity and minimum variation in obliquity, causing a decrease in summer insolation at the poles, which appear to have initiated the expansion of Antarctic ice (43). Overall, the size of the dinoflagellate cyst assemblage is highly correlated with the δ18O record in deep-sea foraminiferal calcite, which is a proxy estimate of changes in deep ocean temperature and ice volume (R = 0.83), and the oceanic temperature gradient (Fig. 1) as inferred from the difference between the δ18O signal in foraminiferal calcite in deep and surface waters (44). Ideally, the vertical gradient in δ18O provides an indication of vertical stratification in the water column; however, the δ18O signal from planktonic foraminifera waters can be diagenetically altered after deposition on the seafloor (45). Hence, surface water temperatures and the vertical temperature gradient may be underestimated. Regardless, the high correlation between the vertical gradient in δ18O and the size of the phytoplankton suggests that climate change and its influence on oceanic circulation may play a role in the macroevolutionary trajectory in cell size in both dinoflagellates and diatoms over geological time scales (Fig. 1).
The macroevolutionary trajectory in the cell size of the dinoflagellate cysts is highly correlated with the change in size in other plankton groups, especially the diatoms (Fig. 1). Coccolith size has not been systematically analyzed through the Cenozoic, but the basic pattern of size variation is certainly broadly similar; there is a post-Cretaceous–Tertiary size minimum, large sizes in the Eocene and Oligocene, then a general decline through the Neogene to a Quaternary minimum (46). Consequently, we suggest that the strong correlation between the median size of the dinoflagellate cysts and climate, in conjunction with similar reported size changes in diatom frustules, coccolith, and planktonic foraminiferal assemblages, indicates that size in the plankton has been actively driven by selection pressures associated with Cenozoic variations in climate. Species diversity in the diatoms generally increases, whereas dinoflagellate cyst diversity generally decreases through the Cenozoic (Fig. 1), yet the median sizes of the dinoflagellate cysts and diatom frustules follow similar patterns throughout the Cenozoic. The lack of coupling between species richness and the median size of the dinoflagellate cysts further indicates that the macroevolutionary size change is an active response to selection pressure rather than a passive response to changes in diversity (Fig. 1). Furthermore, the uncoupling of species richness from ocean temperature indicates that, unlike the planktonic foraminifera (7), oceanic temperature is not a primary determinant of speciation and diversity in the eukaryotic phytoplankton.
There are several potential hypotheses that can explain the mechanistic link between changes in Cenozoic climate, nutrient and light availability in the surface ocean, and the macroevolutionary changes in the size of the plankton. Coincident increases in phosphorus accumulation rates (47), wind speed (48), and vertical stratification in the water column make it difficult to establish how upper mixed-layer depths, light, and nutrient availability have changed through the Cenozoic. If nutrient availability has increased through time (49), then predation (“top-down” control) may explain the macroevolutionary decrease in phytoplankton cell size. Increases in nutrient availability will increase primary production and phytoplankton standing biomass-stimulating increases in the relative abundance of large herbivorous zooplankton. Higher concentrations of prey biomass have been linked to an increase in the abundance of larger consumers that tend to select larger prey, causing a shift in size structure toward smaller prey organisms (50, 51), indicating the relative increase in grazing pressure on the larger phytoplankton species could be the basis of the macroevolutionary decrease in the size of plankton over the Cenozoic. Alternatively, if size is controlled by resource limitation (“bottom-up”), the macroevolutionary decrease in the size of the diatoms and dinoflagellate cysts may reflect a decrease in nutrient availability in the surface ocean because of increased vertical stratification in the Neogene (11), changes in magnitude and frequency of pulses nutrients (52–54), and shifts in the nutrient or environmental conditions limiting primary production through time. In the modern ocean, small cells often dominate assemblages in oligotrophic environments, and larger cells become increasingly abundant as nutrient concentrations increase (39). Although it is likely that a combination of both top-down and bottom-up factors controlled the evolutionary trajectory of plankton size through time, the preponderance of evidence appears to give greater weight to the latter hypothesis.
Materials and Methods
For our analysis, we created a species-level database of the geological ranges and sizes of 275 Cenozoic dinoflagellate cyst species. Species diversity and temporal distribution were determined by merging four published compilations of dinoflagellate cyst occurrences (21, 55–57). Rather than being exhaustive compilations of all described species, these biostratigraphic compilations include only well established and common species, an approach that is useful in avoiding the biases of monographic effects and synonyms. If there was disagreement among sources, the temporal distribution was taken as the oldest first and youngest last occurrence. Stratigraphic ranges used are based on calcareous nannoplankton zones translated into time using a common time scale (58). Taxonomic standardization followed the 1998 Williams et al. index (59).
The sizes of the dinoflagellate fossils were generated from primary descriptions of the species, such as holotype, lectotype, and other secondary descriptions summarized from the primary literature as compiled by J. E. Williams and archived at the Natural History Museum of London. Linear dimensions of length and width were generally provided as minimum, maximum, and average of the inner cyst. Estimates of the linear dimensions of the outer cyst and processes were provided in fewer instances; consequently, only analyses of the area of the inner cyst are presented. To construct the macroevolutionary record of the size of the dominant dinoflagellate cyst community through geological time, the median size and its 95% confidence interval of all species present in each 3 million-year window over the 65-Ma interval was determined. To compare the median size of the dinoflagellate cyst assemblage to paleoenvironmental variables over time, the data were matched to observations with the coarsest temporal resolution, and then reduced major axis regression and the Spearman correlation coefficient were computed. A relationship between cyst volume and volume of the motile cell was assessed by linear regression of laboratory data of extant species, with either calcareous or organic-walled cysts compiled from the literature [references are provided as supporting information (SI)]. To assess the cell volume ratio between dinoflagellates and diatoms for the extant assemblage (0 Ma), cell volumes were estimated by taking the square root of the area and assuming the cells were spheres, and the variation was estimated by using the 95% bootstrapped confidence interval on the median area of the dinoflagellate cysts.
Supplementary Material
ACKNOWLEDGMENTS.
We thank J. E. Williams (Natural History Museum) for indispensable advice and access to his dinoflagellate and palynological library, J. Payne for constructive comments, H. MacDonald for help with Fig. 2, J. Wright for the oxygen isotope record, D. Schmidt for the foraminifera record, and B. van de Schootbrugge for the introduction to S.F.-B. This work was supported by the National Science Foundation (NSF) (Biocomplexity Grant OCE-0084032), NSF Grant OCE 06-23256 (to M.E.K.), and Natural Sciences and Engineering Research Council Discovery Grants (Z.V.F. and A.J.I.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709381104/DC1.
References
- 1.Falkowski PG, Katz ME, Knoll AH, Quigg A, Raven JA, Schofield O, Taylor FJR. Science. 2004;305:354–360. doi: 10.1126/science.1095964. [DOI] [PubMed] [Google Scholar]
- 2.Finkel ZV. Limnol Oceanogr. 2001;46:86–94. [Google Scholar]
- 3.Finkel ZV, Irwin AJ, Schofield O. Mar Ecol Prog Ser. 2004;273:269–279. [Google Scholar]
- 4.Bienfang P, Laws E, Johnson W. J Exp Mar Biol Ecol. 1977;30:283–300. [Google Scholar]
- 5.Munk WH, Riley GA. J Mar Res. 1952;11:215–240. [Google Scholar]
- 6.Oliver MJ, Petrov D, Ackerly D, Falkowski PG, Schofield OM. Genome Res. 2007;17:594–601. doi: 10.1101/gr.6096207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillooly JF, Allen AP, West GB, Brown JH. Proc Natl Acad Sci USA. 2005;102:140–145. doi: 10.1073/pnas.0407735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiorboe T. Adv Mar Biol. 1993;29:1–72. [Google Scholar]
- 9.Moloney CL, Field JG. J Plankton Res. 1991;13:1003–1038. [Google Scholar]
- 10.Azam F, Fenchel T, Field JG, Gray JS, Meyer-Reil LA, Thingstad F. Mar Ecol Prog Ser. 1983;10:257–263. [Google Scholar]
- 11.Finkel ZV, Katz M, Wright J, Schofield O, Falkowski P. Proc Natl Acad Sci USA. 2005;102:8927–8932. doi: 10.1073/pnas.0409907102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderiks J, Rickaby REM. Biogeosciences. 2007;4:323–329. [Google Scholar]
- 13.Schmidt DN, Thierstein HR, Bollmann J, Schiebel R. Science. 2004;207:207–210. doi: 10.1126/science.1090592. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt DN, Renaud S, Bollman J, Schiebel R, Thierstein HR. Mar Micropaleontol. 2004;50:319–338. [Google Scholar]
- 15.Schmidt DN, Lazarus D, Young JR, Kucera M. Earth-Science Rev. 2006;78:239–266. [Google Scholar]
- 16.Head MJ. In: Palynology: Principles and Applications. Jansonius J, McGregor DC, editors. Dallas, TX: American Association of Stratigraphic Palynologist Foundations; 1996. pp. 1197–1248. [Google Scholar]
- 17.Taylor FJR. Ecol Stud Anal Syn. 1973;3:155–169. [Google Scholar]
- 18.Moldowan JM, Talyzina NM. Science. 1998;281:1168–1170. doi: 10.1126/science.281.5380.1168. [DOI] [PubMed] [Google Scholar]
- 19.Fensome RA, MacRae RA, Moldowan JM, Taylor FJR, Williams GL. Paleobiology. 1996;22:329–338. [Google Scholar]
- 20.Fensome RA, Saldarriaga JF, Taylor FJR. Grana. 1999;38:66–80. [Google Scholar]
- 21.Stover LE, Brinkhuis H, Damassa SP, de Verteuil L, Helby RJ, Monteil E, Partridge AD, Powerell AJ, Riding JB, Smelror M, Williams GL. In: Palynology: Principles and Applications. Jansonius J, McGregor DC, editors. Vol 2. College Station, TX: AASP Foundation; 1996. pp. 641–750. [Google Scholar]
- 22.Bujack BJ, Davies EH. Modern and Fossil Peridiniineae, AASP Contribution Series. College Station, TX: AASP; 1983. no 13. [Google Scholar]
- 23.Anderson DM, Coats DW, Tyler MA. J Phycol. 1985;21:200–206. [Google Scholar]
- 24.Olli K, Anderson DM. J Phycol. 2002;38:145–157. [Google Scholar]
- 25.Lewis J, Harris A, Jones K, Edmonds R. J Plankton Res. 1999;21:343–354. [Google Scholar]
- 26.Dawkins R, Krebs JR. Proc R Soc London Ser B. 1979;205:489–511. doi: 10.1098/rspb.1979.0081. [DOI] [PubMed] [Google Scholar]
- 27.Smetacek V. Nature. 2001;411:745. doi: 10.1038/35081210. [DOI] [PubMed] [Google Scholar]
- 28.Frost BW. Limnol Oceanogr. 1972;17:805–815. [Google Scholar]
- 29.Mullin MM. Limnol Oceanogr. 1963;8:239–250. [Google Scholar]
- 30.Parsons TR, LeBrasseur RJ, Fulton JD. J Oceanogr Soc Jpn. 1967;23:10–17. [Google Scholar]
- 31.Irigoien X, Head RN, Harris RP, Cummings D, Harbour D. Limnol Oceanogr. 2000;45:44–54. [Google Scholar]
- 32.Teegarden GJ, Campbell RG, Durbin EG. Mar Ecol Prog Ser. 2001;218:213–226. [Google Scholar]
- 33.Finkel ZV. In: Evolution of Aquatic Photoautotrophs. Falkowksi PG, Knoll AH, editors. San Diego: Academic; 2007. [Google Scholar]
- 34.Jacobson DM. J Eukaryot Microbiol. 1999;46:376–381. [Google Scholar]
- 35.Naustvoll L-J. Phycologia. 2000;39:187–198. [Google Scholar]
- 36.Naustvoll L-J. Phycologia. 2000;39:448–455. [Google Scholar]
- 37.Rigby S, Milsom CV. Annu Rev Ecol Syst. 2000;31:293–313. [Google Scholar]
- 38.Loeuille N, Loreau M. Proc Natl Acad Sci USA. 2005;102:5761–5766. doi: 10.1073/pnas.0408424102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irwin AJ, Finkel ZV, Schofield OME, Falkowski PG. J Plankton Res. 2006;28:459–471. [Google Scholar]
- 40.Furnas MJ. J Plankton Res. 1990;12:1117–1151. [Google Scholar]
- 41.Tang EPY. J Phycol. 1996;32:80–84. [Google Scholar]
- 42.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Science. 2001;292:686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- 43.Zachos JC, Shackelton NJ, Revenaugh JS, Palike H, Flower BP. Science. 2001;292:274–278. doi: 10.1126/science.1058288. [DOI] [PubMed] [Google Scholar]
- 44.Wright JD. In: Encyclopedia of Ocean Sciences. Steele J, Thorpe S, Turekian K, editors. London: Academic; 2001. pp. 415–426. [Google Scholar]
- 45.Pearson PN, Ditchfield PW, Singano J, Harcourt-Brown KG, Nicholas CJ, Olsson RK, Shackelton NJ, Hall MA. Nature. 2001;413:481–487. doi: 10.1038/35097000. [DOI] [PubMed] [Google Scholar]
- 46.Bown PR, Dunkley-Jones T, Lees JA, Randell R, Coxall H, MacMillan I, Mizzi J, Pearson P, Wade B, Young JR. Bull Geol Soc Am. 2007 in press. [Google Scholar]
- 47.Follmi KB. Geology. 1995;23:859–862. [Google Scholar]
- 48.Rea DK. Rev Geophys. 1994;32:159–195. [Google Scholar]
- 49.Bambach RK. Paleobiology. 1993;19:372–397. [Google Scholar]
- 50.Brooks JL, Dodson SI. Science. 1965;150:28–35. doi: 10.1126/science.150.3692.28. [DOI] [PubMed] [Google Scholar]
- 51.Bergquist AM, Carpenter SR, Latino JC. Limnol Oceanogr. 1985;30:1037–1045. [Google Scholar]
- 52.Grover JP. Am Nat. 1991;138:811–835. [Google Scholar]
- 53.Tozzi S, Schofield O, Falkowski P. Mar Ecol Prog Ser. 2004;274:123–132. [Google Scholar]
- 54.Falkowski PG, Oliver MJ. Nat Rev Microbiol. 2007;5:813–819. doi: 10.1038/nrmicro1751. [DOI] [PubMed] [Google Scholar]
- 55.Powell AJ. In: A Stratigraphic Index of Dinoflagellate Cysts. Powell AJ, editor. Cambridge, UK: Chapman & Hall; 1992. pp. 155–252. [Google Scholar]
- 56.Harland R. In: A Stratigraphic Index of Dinoflagellate Cysts. Powell AJ, editor. Cambridge, UK: Chapman & Hall; 1992. pp. 253–273. [Google Scholar]
- 57.Williams GL, Brinkhuis H, Pearce MA, Fensome RA, Weegink JW. In: Proceedings of the Ocean Drilling Program: Scientific Results. Exon NF, Kennett JP, Malone MJ, editors. Vol 189. College Station, TX: Ocean Drilling Program; 2004. pp. 1–98. [Google Scholar]
- 58.Berggren WA, Kent DV, Seisher CC, III, Aubry MP. In: Geochronology, Time Scales and Global Stratigraphic Correlation, SEPM Special Publication Series. Berggren WA, Kent DV, Aubry MP, Hardenbol J, editors. Vol 54. Tulsa, OK: SEPM; 1995. pp. 129–212. [Google Scholar]
- 59.Williams GL, Lentin JK, Fensome RA. The Lentin and Williams Index of Fossil Dinoflagellates. College Station, TX: AASP Foundation, Texas A&M University; 1998. [Google Scholar]
- 60.Tang EPY. J Plankton Res. 1995;17:1325–1335. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.