Abstract
Chemokine receptors regulate the trafficking of leukocytes by mediating chemotaxis and by their influence on the expression and/or affinity of leukocyte integrins. Using blocking mAb, we showed that antigen-induced recruitment of mast cell progenitors (MCp) to the lung requires interaction of a4 integrins on the MCp with endothelial vascular cell adhesion molecule 1 (VCAM-1). In seeking a chemokine component, we found that CXCR2-deficient but not CCR3- or CCR5-deficient sensitized and antigen-challenged mice have significantly fewer lung MCp 1 day after challenge and fewer tracheal intraepithelial MC 1 week after challenge, implying that recruited MCp provide the source for these mature MC. Unexpectedly, reconstitution of sensitized, sublethally irradiated +/+ and −/− mice with bone marrow cells of either genotype indicated that expression of CXCR2 by the migrating MCp was not required. Instead, receptor function by resident lung cells was required because normal BM did not reconstitute MCp recruitment in irradiated CXCR2−/− mice. The reduced MCp influx into the lung of CXCR2−/− mice was accompanied by reduced induction of VCAM-1 transcripts and reduced endothelial surface expression. Thus, these studies demonstrate a role for a chemokine receptor in regulating endothelial VCAM-1 expression, MCp migration, and the level of intraepithelial MC in the lung of aerosolized, antigen-challenged mice.
Keywords: chemokine receptors, endothelium, lung inflammation
The bronchial inflammation in atopic asthma and in allergen-induced mouse models of pulmonary inflammation is characterized by the influx of various effector cell populations including Th2 cells, eosinophils, basophils, and mast cells (MC). The MC numbers increase both in the smooth muscle and in the epithelium of the airways of humans with atopic asthma (1, 2). Moreover, there is marked hyperplasia of intraepithelial (IE) MC in mice with antigen-induced Th2-mediated inflammation of the airways, although normal mouse lung lacks appreciable numbers of MC (3, 4). That antigen-induced airways hyperreactivity is diminished in MC-deficient strains indicates the importance of the MC to the biology of the host response (3, 5).
Recruitment of MC progenitors (MCp) to the lung of sensitized, aerosolized antigen-challenged BALB/c mice is rapid and depends on expression of α4β1 or α4β7 integrins because this response was blocked by mAb to these integrins, with the counterligand being vascular cell adhesion molecule 1 (VCAM-1) (6). Because transendothelial migration of cells into tissues characteristically involves not only adhesion but also directed migration, we turned our attention to possible chemokine receptors on the MCp. Using null mouse strains lacking CCR3, CCR5, or CXCR2, which are known to be expressed by cultured mouse bone marrow-derived MC (mBMMC) and/or by human cord blood-derived MC (7–9), we investigated the requirement for these receptors on MCp recruitment to antigen-sensitized and challenged mice. We find that MCp recruitment to the lung of sensitized, BALB/c mice after three daily aerosolized antigen challenges depends on CXCR2 function in the lung parenchyma/microvasculature rather than on the MCp with selectivity for the MCp relative to other mononuclear cells. The deficiency in CXCR2 is associated with a reduction in VCAM-1 expression in the lung and with a reduction in the subsequent appearance of mature mucosal MC in the trachea, suggesting that the recruitment is a prerequisite for the IE MC hyperplasia.
Results
Reduced MCp Recruitment to Lung of Sensitized and Antigen-Challenged CXCR2−/− Mice.
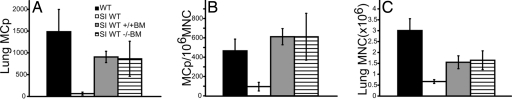
We demonstrated that MCp recruitment to the lung of ovalbumin (OVA)-sensitized and aerosolized OVA-challenged BALB/c mice approaches a plateau after only three daily challenges (6). To evaluate any effect resulting from the loss of expression of a chemokine receptor, the numbers of mononuclear cells (MNC) and MCp in the lung of sensitized, antigen-challenged wild-type (WT) (BALB/c) and CXCR2-null mice were compared. Sensitized WT and CXCR2−/− mice that were not challenged with aerosolized antigen show similar low levels of MNC and MCp in the lung as noted previously (Fig. 1) (6, 10). After three challenges, sensitized, antigen-challenged WT mice (n = 8) showed a 13-fold increase in the absolute number of lung MCp per mouse, whereas sensitized, antigen-challenged CXCR2−/− mice (n = 8) showed only a 3-fold increase. This result represents a statistically significant reduction in MCp recruitment of 66 ± 7% (mean ± SE) in the CXCR2−/− strain. A significant reduction of 53 ± 12% (mean ± SE) was also noted in the concentration of MCp per 106 MNC isolated from the lung (Fig. 1B), whereas the influx of MNC was not different between the deficient and sufficient strains (Fig. 1C), indicating that there is a selective reduction in MCp within the MNC population. Analyses of the percentage of CD3+, CD4+, CD8+, and CD19+ cells within the MNC populations isolated from sensitized, antigen-challenged WT and CXCR2−/− mice were not different (data not shown).
Fig. 1.
MCp recruitment to the lung is reduced in antigen-challenged CXCR2−/− mice. (A) Total number of lung MCp per mouse from OVA-sensitized WT (black bars) and CXCR2−/− (open bars) mice with and without aerosolized OVA challenges. Values are the mean ± SE from eight determinations in seven separate experiments. (B) Concentration of MCp (MCp per 106 MNC) in the lungs of the same mice. (C) Number of lung MNC recovered per mouse in the same mice. The asterisk (*) indicates statistical significance (P < 0.05) as determined by a two-tailed Student's t test.
We also evaluated mice lacking the β chemokine receptors CCR3 and CCR5. Similar numbers of total lung MCp per mouse and MCp/106 MNC and lung MNC per mouse were found in unchallenged CCR3−/− mice compared with BALB/c controls and in unchallenged CCR5−/− mice compared with B6129F2 controls. Similar increases in all three measurements were observed in both sensitized, antigen-challenged null strains compared with their respective sensitized, antigen-challenged WT mice treated and analyzed in parallel [supporting information (SI) Table 2]. These results indicate that neither of these chemokine receptors is involved in MCp recruitment to allergen-challenged lung.
WT and CXCR2−/− Mice Show Similar Levels of Serum Ig and Cellular Infiltration of the Bronchovascular Bundles with Sensitization and Antigen Challenge.
A previous study with CXCR2-deficient mice found that the total and OVA-specific IgE levels were increased significantly after seven daily OVA-aerosol challenges (11). With our protocol, total and OVA-specific IgE levels in sensitized, antigen-challenged CXCR2-deficient mice were not significantly different from the WT controls, 2.3 ± 0.4 vs. 3.1 ± 1.1 μg/ml, respectively, for total IgE (n = 9 and n = 8), and 56 ± 28 vs. 26 ± 6 ng/ml respectively, for OVA-specific IgE (n = 9 for both strains). Total IgG1 levels were similar for both strains, 3.1 ± 0.78 mg/ml (sensitized, antigen-challenged WT) vs. 3.5 ± 0.97 mg/ml (sensitized, antigen-challenged CXCR2−/−) (n = 5 for each strain). Total IgG2a levels were higher for sensitized, antigen-challenged CXCR2−/− (all >100 μg/ml) than in sensitized, antigen-challenged WT (75 ± 9 μg/ml) (n = 5 for each strain).
Histological evaluation of sensitized, antigen-challenged WT and CXCR2−/− lung showed similar levels of inflammation of the bronchovascular bundles both 1 day and 1 week after the last antigen challenge. On day 20, 1 day after the last challenge, sensitized, antigen-challenged WT mice had 37 ± 12% (mean ± SE, n = 9) of the blood vessels and 15 ± 5% of the bronchioles with an associated inflammatory infiltrate and sensitized, antigen-challenged CXCR2−/− mice had 34 ± 12% (n = 7) of the blood vessels and 13 ± 6% of the bronchioles with an associated infiltrate (Table 1). There was no significant influx of neutrophils among the infiltrating cells in either strain at this time point. One week after the last challenge, the inflammation scores were comparable with the scores on day 20 and not significantly different between the groups. Unchallenged mice of both genotypes showed no signs of lung inflammation.
Table 1.
Histological evaluation of lung inflammation in sensitized, antigen-challenged WT and CXCR2−/− mice
| Inflammation | Day 20 |
Day 27 |
||||
|---|---|---|---|---|---|---|
| Mean | SE | n | Mean | SE | n | |
| Blood vessels | ||||||
| BALB/c | 37 | 12 | 9 | 38 | 8 | 11 |
| CXCR2−/− | 34 | 12 | 7 | 38 | 9 | 12 |
| Bronchioles | ||||||
| BALB/c | 15 | 5 | 9 | 18 | 5 | 11 |
| CXCR2−/− | 13 | 6 | 7 | 17 | 5 | 12 |
The values are the mean percentage of inflamed blood vessels and bronchioles in sensitized, antigen-challenged BALB/c and CXCR2−/− mice evaluated 1 day (day 20) and 1 week (day 27) after challenge. The number of animals in each group is listed under the column heading n.
CXCR2−/− BM Reconstitutes Recruitment of MCp to the Lung of Sensitized, Sublethally Irradiated, Antigen-Challenged WT Mice.
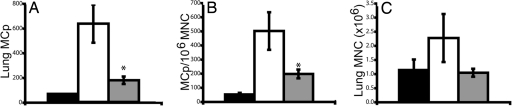
To evaluate whether the decreased recruitment of MCp to inflamed lung in the CXCR2−/− mice is caused by the loss of expression of CXCR2 on the MCp, we reconstituted sensitized, sublethally irradiated WT mice with WT or CXCR2−/− bone marrow (BM) and then challenged the mice 1 week later. Sensitized, sublethally irradiated, antigen-challenged WT mice that were not reconstituted with BM before challenge have very few lung MCp, a reduced concentration of MCp per 106 MNC, and reduced numbers of lung MNC per mouse compared with nonirradiated, sensitized, antigen-challenged WT mice (Fig. 2). Reconstitution of sensitized, sublethally irradiated, antigen-challenged WT mice with WT BM restored the recruitment of MCp to the lung; the total lung MCp per mouse was >10-fold that of nonreconstituted mice. The total influx of lung MCp per mouse represents 61% (mean, n = 4 mice) of the level seen in the nonirradiated, sensitized, antigen-challenged WT controls. It also restored the concentration of MCp per 106 MNC to 131% of challenged unirradiated controls, although fewer total lung MNC per mouse were obtained. Sensitized, sublethally irradiated, antigen-challenged WT mice that were reconstituted with CXCR2−/− BM showed virtually identical responses in the number of total lung MCp recruited per mouse, concentration of MCp per 106 MNC, and in the number of lung MNC per mouse compared with similarly treated WT mice reconstituted with WT BM in parallel (Fig. 2). These results indicate that CXCR2 expression by the MCp is not critical to this response, and they suggest that CXCR2 needs to be expressed on a non-BM-derived radiation-resistant cell in the lung parenchyma.
Fig. 2.
CXCR2−/− BM reconstitutes MCp recruitment to the lung of sensitized, sublethally irradiated, antigen-challenged WT mice. (A) Total number of lung MCp per mouse from sensitized, antigen-challenged WT mice (black bar), from sensitized, sublethally irradiated, antigen-challenged WT mice without BM reconstitution (SI WT; open bar), from sensitized, sublethally irradiated, antigen-challenged, WT mice reconstituted with WT BM (SI WT +/+BM; gray bar), and from sensitized, sublethally irradiated, antigen-challenged WT mice reconstituted with CXCR2−/− BM (SI WT −/−BM; striped bar). Values are the mean ± SE from four mice in two separate experiments. (B) Concentration of MCp (MCp per 106 MNC) in the lungs of the same mice. (C) Number of lung MNC recovered per mouse in the same mice.
WT BM Does Not Reconstitute MCp Recruitment to the Lung of Sensitized, Sublethally Irradiated, Antigen-Challenged CXCR2−/− Mice.
To determine whether the functional expression of CXCR2 by a radiation-resistant lung parenchymal cell could account for the reduced MCp recruitment to the lung in sensitized, antigen-challenged CXCR2−/− mice, recruitment was evaluated in sensitized, sublethally irradiated, antigen-challenged CXCR2−/− and in similarly treated WT mice reconstituted with WT BM. Compared with sensitized, sublethally irradiated, antigen-challenged WT mice reconstituted with WT BM, similarly treated CXCR2−/− mice reconstituted with the WT BM cells had a significant 75 ± 8% (P < 0.05, n = 7 mice) reduction in the total number of lung MCp per mouse, a significant 53 ± 11% (P < 0.05) reduction in the concentration of MCp per 106 MNC, and a 49 ± 21% reduction of the number of lung MNC per mouse (Fig. 3). Moreover, in a separate experiment, we found that the genotype of the donor BM did not influence recruitment to the lung of sensitized, sublethally irradiated, antigen-challenged CXCR2−/− mice. Sensitized, sublethally irradiated, antigen-challenged CXCR2−/− mice reconstituted with WT or with CXCR2−/− BM had 259 ± 17 (73% reduction, n = 2) and 227 ± 77 (76% reduction, n = 2) total lung MCp per mouse, respectively whereas similarly treated WT mice reconstituted with WT BM had 951 ± 111 total lung MCp per mouse (mean ± half-range, n = 2).
Fig. 3.
WT BM does not reconstitute MCp recruitment to the lung of sensitized, sublethally irradiated, antigen-challenged CXCR2−/− mice. (A) Total number of lung MCp per mouse from sensitized, sublethally irradiated, antigen-challenged WT mice without BM reconstitution (black bar), from sensitized, sublethally irradiated, antigen-challenged WT mice reconstituted with WT BM (open bar) and from sensitized, sublethally irradiated, antigen-challenged CXCR2−/− mice reconstituted with WT BM (gray bar). Values are the mean ± SE from six WT and seven CXCR2−/− mice in three separate experiments, except for the no reconstitution control, which was done in two of the three experiments. (B) Concentration of MCp (MCp per 106 MNC) in the lungs of the same mice. (C) Number of lung MNC recovered per mouse in the same mice. The asterisk (*) indicates statistical significance (P < 0.05) as determined by a two-tailed Student's t test.
Reduced VCAM-1 Transcription and Expression on Endothelium in the Lung of Sensitized, Antigen-Challenged CXCR2−/− Mice.
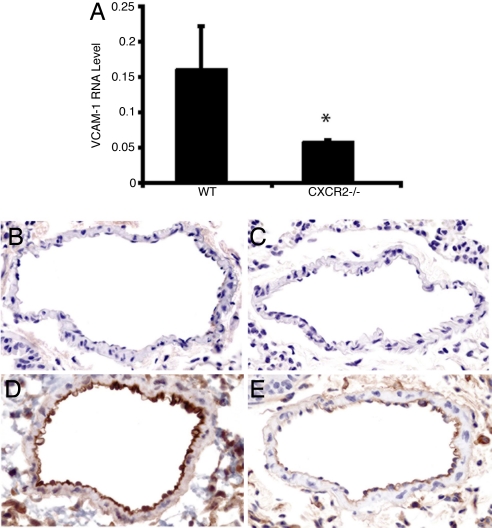
Lung endothelial cells express both CXCR2 and the ligand for α4 integrins, VCAM-1 (12–14). Blocking of either α4 integrins or VCAM-1 with monoclonal antibody suppresses antigen-induced MCp recruitment to lung (6). To test the possibility that endothelial VCAM-1 up-regulation was impaired in CXCR2-deficient mice, we evaluated the level of VCAM-1 mRNA in antigen-challenged lung by real-time RT-PCR (relative to GAPDH expression). The expression of VCAM-1 mRNA in sensitized, antigen-challenged CXCR2−/− lung 24 h after the last challenge, when MCp influx is assessed, was significantly reduced (54% reduction, P < 0.01) relative to the level in sensitized, antigen-challenged WT lung (n = 7 WT and 6 CXCR2−/− mice) (Fig. 4A). In contrast, there was no difference in the level of expression of mRNA for the MC growth and chemotactic factor, stem cell factor, or for TNF-α (data not shown). Analysis of VCAM-1 transcripts at 1, 6, or 24 h after challenges indicated that the transcript level increased over time, reaching significance in sensitized, antigen-challenged WT mice at the 24-h time point (2-fold induction, P < 0.05; data not shown). In sensitized and antigen-challenged CXCR2−/− mice, there was no change in expression levels over this period. To establish that the reduction in the level of VCAM-1 transcript was associated with a reduction in VCAM-1 protein, we evaluated its expression on the endothelium by immunohistochemistry. Sensitized, unchallenged WT (Fig. 4B) and CXCR2−/− (Fig. 4C) mice showed no or very weak staining for VCAM-1 (n = 4 for both genotypes). With sensitization and antigen challenge, endothelial cell expression of VCAM-1 in WT mice was strong (Fig. 4D), whereas sensitized, antigen-challenged CXCR2−/− mice (Fig. 4E) exhibited much less expression of VCAM-1 on the endothelium (n = 4 for both genotypes). Quantification of the density of the immunostaining demonstrated a consistently lower level of induced VCAM-1 expression over background in sensitized, antigen-challenged CXCR2−/− mice compared with the similarly treated WT within each of the two experiments (SI Fig. 6).
Fig. 4.
VCAM-1 transcripts in lung and protein expression on lung endothelium are reduced in sensitized, antigen-challenged CXCR2−/− mice. (A) Relative VCAM-1 mRNA expression (compared with GAPDH) in lung from sensitized, antigen-challenged WT (Left) and sensitized, antigen-challenged CXCR2−/− (Right) mice 1 day after challenge (n = 7, from two separate experiments). The asterisk (*) indicates statistical significance (P < 0.05), as determined by a two-tailed Student's t test. (B–E) Representative pictures (of four mice of each genotype in two separate experiments) of VCAM-1 expression on lung endothelium in sensitized WT mice (B), sensitized CXCR2−/− mice (C), sensitized, antigen-challenged WT mice (D), and sensitized, antigen-challenged CXCR2−/− mice (E).
Reduced Numbers of IE MC in the Trachea of Sensitized, Antigen-Challenged CXCR2−/− Mice.
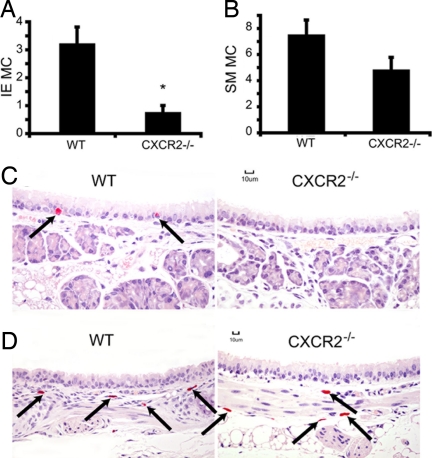
OVA-sensitized but unchallenged mice have no IE MC in the trachea (n = 4, results not shown). One day after challenge, only a few mature IE MC were seen in the tracheas of sensitized, antigen-challenged WT mice, and none were detected in sensitized, antigen-challenged CXCR2−/− mice (0.4 ± 0.2 vs. 0 ± 0 MC per cross-section of trachea, respectively, mean ± SE, n = 9 WT and 7 CXCR2−/− mice). One week after challenge there was an increase in mature IE MC in both genotypes, 3.2 ± 0.6 vs. 0.7 ± 0.3, respectively (mean ± SEM, n = 9 WT and 10 CXCR2−/− mice). The number of IE MC in sensitized, antigen-challenged WT increased by 8-fold and was significantly (P < 0.05) greater than in sensitized, antigen-challenged CXCR2−/− mice. The appearance of IE MC in sensitized, antigen-challenged WT mice and not in CXCR2−/− mice is illustrated in Fig. 5 A and C. The numbers of tracheal submucosal MC were similar in both genotypes at 1 day after challenge (5 ± 0.9 vs. 7 ± 2, respectively, n = 5 for both groups) and not significantly different in either genotype 1 week after challenge (Fig. 5 B and D).
Fig. 5.
The number of tracheal IE MC is reduced in sensitized, antigen-challenged CXCR2−/− mice. (A) The mean (±SE) number of IE MC per tracheal cross-section in sensitized, antigen-challenged WT and sensitized, antigen-challenged CXCR2−/− mice 1 week after challenge. (B) The mean (±SE) number of submucosal (SM) MC per tracheal cross-section in sensitized, antigen-challenged WT and CXCR2−/− mice 1 week after challenge. The asterisk (*) indicates statistical significance (P < 0.05, n = 9 WT and 10 CXCR2−/−, from two separate experiments) as determined by a two-tailed Student's t test. (C) Representative images of tracheal epithelium demonstrating IE MC (arrows) in sensitized, antigen-challenged WT but not in the sensitized, antigen-challenged CXCR2−/− mice. (D) Representative images of tracheal SM MC (arrows) in sensitized, antigen-challenged WT and sensitized, antigen-challenged CXCR2−/− mice. (Scale bars: 10 μm.)
Discussion
These findings demonstrate a pathway for the regulation of the recruitment of MCp to inflamed lung in which the CXCR2-regulated expression of VCAM-1 on endothelium plays a key role. This result expands the paradigm of the action of chemokine receptors in directing leukocyte migration beyond directed migration along a chemokine gradient. It also highlights the active role played by the endothelium in the process of diapedesis.
We initiated studies on the recruitment of MC to the lung with aerosolized antigen challenge to test the hypotheses that the expansion of pulmonary MC that accompanies allergic inflammation (1–4) involves recruitment as opposed to expansion of the indigenous population. The initial studies demonstrated the antigen-induced recruitment of MCp to lung of sensitized mice and showed that this process used the interaction of the α4 integrins on the progenitors and VCAM-l on the lung tissue (6). There have been no studies addressing the role of chemokines and their receptors in the recruitment of MCp to the lung, although our studies of the constitutive homing of MCp to the intestine of naive mice showed a role for CXCR2 as well as α4β7 integrins and VCAM-1 or mucosal addressin CAM (MAdCAM)-1 (10, 15). Therefore, we assessed the role of several chemokine receptors in the pulmonary recruitment of MCp by antigen challenge of sensitized mice. The recruitment of MCp with antigen challenge was significantly diminished in CXCR2-deficient mice compared with their sufficient controls, whereas CCR3- and CCR5-deficient mice exhibited normal recruitment of MCp to the lung. WT mice subjected to sublethal irradiation were fully reconstituted with CXCR2-deficient or sufficient BM, indicating that the functional CXCR2 was not on the BM-derived leukocytes. When the sublethal irradiation was directed to the CXCR2-deficient and sufficient strains, adoptive transfer of WT BM failed to reconstitute the deficient recipient, revealing that the dominant CXCR2 function resided with resident lung cells.
MCp influx is specific migration and not merely disruption of tight junctions because the migration is blocked by antibodies to the α4 integrins or VCAM-1 (6, 10, 15). Of interest, expression of the latter adhesion molecule has been linked to CXCR2 signaling in endothelial cells. CXCR2–ligand interactions induce NF-κB activation via PI3K in rat cardiac-derived endothelial cells, and inhibition of NF-κB in a bronchial epithelial cell line or in human umbilical cord endothelial cells suppresses VCAM-1 expression (16–18). Because VCAM-1 was a critical counterligand in the pulmonary vasculature for the α4 integrins on MCp, we established that sensitized, antigen-challenged CXCR2-deficient mice had significantly less VCAM-1 RNA and protein expression on the endothelium of the lung than similarly treated WT mice. TNF-α, which induces VCAM-1 expression in endothelium, was unimpaired in sensitized, antigen-challenged CXCR2−/− mice as was also true for the MC active cytokine stem cell factor. Thus, the primary mechanism for the reduction in VCAM-1 is not a change in the expression of TNF-α but more likely the absence of CXCR2 signaling.
Importantly, in our protocol in which we assess the changes at a very early time point, the intact IgE response and bronchovascular inflammation in the CXCR2−/− mice indicate that the impaired MCp recruitment is relatively selective. This selectivity of lung CXCR2 for MCp recruitment in our work is supported by the additional findings of the intact MNC yields from dissociated lung in sensitized, antigen-challenged CXCR2−/− mice and by the similar numbers of the various subclasses of lymphocytes found in the MNC population isolated from the two strains. In other models, CXCR2 deficiency has been implicated in the influx of both neutrophils and T cells (11, 14, 19–23). In a study of the OVA-induced pulmonary response, De Sanctis et al. (11) showed that OVA-sensitized CXCR2-deficient mice subjected to a single OVA-aerosol challenge had diminished neutrophil recruitment and an increase in B cells in the lung after multiple challenges. Reutershan et al. (14) also found that neutrophil recruitment after LPS-induced lung injury depended in part on the expression of CXCR2 by the neutrophil. In contrast, using a more chronic model of Aspergillus-induced inflammation, Schuh et al. (20) noted that the influx of eosinophils and T cells was reduced in CXCR2-null mice but that there was no effect on neutrophil recruitment. Because we do not see an influx of neutrophils into the lung of WT mice after three daily challenges or any change in various T cell populations, we attribute the other cellular effects reported in the literature to differences in protocols for the pathobiology and the complexity of CXCR2 signaling in either the BM cells being recruited, the resident tissue being infiltrated, or some combination.
One week after challenge, the CXCR2−/− mice had significantly fewer IE MC than the WT mice treated in parallel. The similar degree of decrease in recruited MCp in the CXCR2−/− mice observed 1 day after challenge suggests that these cells provide the basis for the increase in the number of mature IE MC seen 1 week later. There was no difference in the submucosal MC of the trachea in the two strains at any time point analyzed, before challenge, at 1 day after challenge, or at 1 week after challenge, indicating that these cells are not significantly affected within this time frame. However, because CXCR2 also is expressed by pulmonary epithelium (14), we cannot ruled out a secondary effect on either maturation or migration into the epithelial compartment.
Altogether, these results reveal that the loss of expression of CXCR2 on the airway endothelium is critical to the specific antigen-induced recruitment of MCp to the lung and suggest that the decreased recruitment is caused by CXCR2 controlling VCAM-1 expression on the endothelium. Furthermore, the findings suggest that regulation of the influx of MCp to the lung ultimately controls the level of IE MC. This action of chemokines on the endothelium highlights a pathway whereby the chemokines regulate leukocyte trafficking by means other than affecting directed migration.
Materials and Methods
Animals.
BALB/c mice were obtained from Taconic Laboratories or The Jackson Laboratory. CXCR2−/− mice were obtained from The Jackson Laboratory, and heterozygotes were intercrossed in our facility to obtain CXCR2−/− and WT mice. CCR5−/− mice and their WT controls (B6129F2) were obtained from The Jackson Laboratory, whereas the CCR3−/− mice and their WT controls (BALB/c) were provided by Craig Gerard (Children's Hospital, Boston). Mice were 6–16 weeks old when used, and the genotype of the CXCR2−/− offspring was determined by PCR as described in ref. 19. All animal experiments were approved by the Institutional Animal Care and Use Committee, and the studies were carried out in accordance with the guidelines for animal care of the National Institutes of Health and the Public Health Service.
OVA Sensitization Protocol.
As described in ref. 6, mice were sensitized i.p. with of 10 μg of OVA (A5503; Sigma–Aldrich) adsorbed to 1 mg of alum (77161; Pierce) in 200 μl of sterile HBSS on day 0 and day 7. Mice were challenged with 1% aerosolized OVA in HBSS for 30 min/day by using a PARI nebulizer on days 17–19 and killed for MCp analysis on day 20. In some experiments, mice were rested for 1 week after challenge and then were killed on day 27 for analysis of mature MC.
MNC Preparation and MCp Assessment.
Mice were killed by CO2 asphyxiation, and both lung and spleen were harvested. Lung and spleen were placed separately in 20 ml of RPMI 1640 complete medium [RPMI 1640 containing 100 units/ml penicillin, 100 μg/ml streptomycin, 10 μg/ml gentamicin, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 1 mM pyruvate, 10 mM Hepes, 5 × 10−5 M 2-mercaptoethanol, and 10% heat-inactivated FCS (F2442; Sigma–Aldrich)] and were processed to obtain the MNC populations that are used to determine the number of MCp by limiting dilution as described (6, 15, 24, 25). The MCp concentration within the MNC population is expressed as the number of MCp per 106 MNC isolated from the tissue. Multiplying the concentration of MCp by the MNC yield for each mouse gives the number of lung MCp per mouse. Numbers were calculated for individual mice, and the values presented are the means (±SE) of values from various numbers of individual mice as indicated.
To establish whether the MCp detected by the liming dilution analysis in the presence of SCF and IL-3 were committed MCp or myeloid progenitor cells driven by our culture conditions, we analyzed the MC lineage progenitors in MNC from lung of sensitized, antigen-challenged WT mice by both flow cytometry and the limiting dilution analysis in parallel. As described, committed MCp are characterized as lineage marker−, CD34+, c-Kit+, and β7 integrinhi cells, whereas myeloid cells not yet committed to the MC lineage are c-Kit+ but express low or no β7 integrin (26). In the lineage marker− population of lung MNC (lineage markers were CD3, CD4, CD8, CD19, B220, and Gr1), the numbers of CD45+, CD34+, FcεRI+, and β7hi cells were similar in percentage to the concentration of MCp obtained from the liming dilution analysis in two separate experiments: 0.022% and 0.029%, respectively, and 0.042% and 0.047%, respectively. These data indicated that the majority of the cells being detected by the limiting dilution assay were committed MCp.
Reconstitution of Recruitment by Adoptive Transfer of BM.
For reconstitution of MCp recruitment in sensitized, antigen-challenged mice, the mice were immunized on day 0 and day 7, sublethally irradiated on day 14 with 500 rad, and then reconstituted with 15 × 106 BM cells 2–4 h later. The BM cells were flushed from the femur and tibia of donor mice with RPMI 1640 complete medium, spun down, counted, and kept on ice at a concentration of 75 × 106 cells per ml until transfer into the tail vein of the recipient mice. The reconstituted mice were challenged with 1% aerosolized OVA on days 21–23, and the determination of MCp was performed on day 24.
Determination of Total and OVA-Specific IgE, Total IgG1, and Total IgG2a.
The levels of total and OVA-specific IgE as well as total IgG1 and IgG2a in the serum were assayed by ELISA. Blood samples were collected by cardiac puncture after mice were sedated by pentobarbital (Sigma–Aldrich) injection, and serum was separated by centrifugation. Total IgE, IgG1 and IgG2a were each determined with the appropriate mouse ELISA kit (BD PharMingen) according to the manufacturer's protocol. To determine OVA-specific IgE, purified rat anti-mouse IgE mAb (BD PharMingen) was used as a capture antibody, and biotinylated OVA was used as a detection reagent (EZ biotin quantitation kit; Pierce). The standard for OVA-specific IgE is hyperimmune BALB/c serum originally standardized by using a monoclonal IgE kindly provided by L. Kobzik (Harvard School of Public Health, Boston).
RNA Preparation and Real-Time RT-PCR.
One lobe of lung from each mouse was homogenized in 1 ml of TRI reagent (T9424; Sigma–Aldrich) by using a tissue homogenizer. RNA was isolated according to the manufacturer's instructions and stored in sterile water at −70°C. cDNA was prepared by using the Iscript cDNA synthesis kit (170-8891; Bio-Rad). Analysis of the amount of a particular transcript was performed by real-time RT-PCR on a Stratagene MX3000P PCR machine by using GAPDH as an internal standard. Validated primers for VCAM-1 and GAPDH were purchased from Qiagen. Primers for comparing GAPDH to TNF-α and stem cell factor were ordered from Sigma–Genosys based on published sequences (27) and analyzed by using ABI SYBR Green QPCR master mix kit from Stratagene (600548) with the following conditions: 15 min, 95°C, then 40 cycles of 15 s at 95°C, 1 min at 60°C, and 30 s at 72°C, followed by 1 min at 95°C, 30 s at 55°C, and 30 s at 95°C.
Histology.
The assessment of inflammation in bronchovascular bundles and the assessment of the number of tracheal MC were done on day 20 or day 27 (1 day or 1 week after the last of three daily challenges). Inflammation in the lung parenchyma was evaluated as described (6, 28) by counting the number of bronchovascular bundles with an inflammatory infiltrate out of a total of 15–20 bundles randomly visualized in each section. A mean percentage of inflamed vessels per mouse was then used to calculate the mean percentage of inflamed vessels per group. MC numbers were evaluated by counting the number of MC in three to five tracheal sections per mouse in the submucosa or in the epithelium. This number was then used to calculate the mean number of IE or submucosal MC per tracheal cross-section per group. MC are distinguished from neutrophils by their more intense reactivity with chloroacetate esterase and mononuclear appearance. The slides were analyzed with a Leica DM LB2 microscope (Leica Microsystems) and a Leica HCX PL Fluotar ×40/0.75 objective lens. The pictures were taken with a Nikon digital camera DXM 1200 with C-0.63 x DXM relay lens. The acquisition software was Nikon ACT-1 version 2.70.
Immunohistochemistry.
The large lobe of the right lung was fixed in 10% neutral buffered formalin (Fischer Scientific) embedded in paraffin. Antigen retrieval was performed with 10 μM trypsin in PBS at 37°C for 30 min. VCAM-1, mAb (clone H-276) (sc-8304; Santa Cruz Biotechnology) was diluted 1:800 in PBS and detected by using the rabbit ABC staining system (sc-2018; Santa Cruz) according to the instructions from the manufacturer. The semiquantitative assessment of the endothelial VCAM-1 expression was performed by using five similar-sized blood vessels for each mouse. The intensity of staining was obtained by selective analysis the endothelium. The image color was converted to gray scale and the relative intensity determined by using ImageJ (National Institutes of Health). The scale used sets white = 0 and black = 255.
Flow Cytometry.
Cell surface phenotypes of lung MNC were analyzed by flow cytometry on a BD Biosciences FACS Canto. The staining and analysis were performed as described in ref. 26. Antibodies used were phycoerythrin (PE)-conjugated anti-mouse CD3 (catalog no. 01085A), PE-cy7-conjugated anti-mouse CD19 (catalog no. 552854), FITC-conjugated anti-mouse CD8a (catalog no. 01044A0), PE-conjugated rat anti-mouse CD4 (catalog no. 01065b), and the appropriate isotype controls all from BD–PharMingen.
Statistical Analysis.
Data were expressed as the mean ± SE for values derived from individual mice analyzed in two or more experiments. Significance was determined by using a two-tailed Student's t test. Values of P < 0.05 were considered significant.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Lena G. Liu for help with immunohistochemistry, Dr. Craig Gerard for providing the CCR3-deficient mice, and Dr. Joshua Boyce for many fruitful discussions. This work was supported by National Institutes of Health Grants AI031599, HL036110, AI057991, and HD028827 and by a grant from the Swedish Society for Medical Research (to J.H.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709651104/DC1.
References
- 1.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 2.Pesci A, Foresi A, Bertorelli G, Chetta A, Olivieri D. Am Rev Respir Dis. 1993;147:684–689. doi: 10.1164/ajrccm/147.3.684. [DOI] [PubMed] [Google Scholar]
- 3.Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. J Clin Invest. 2006;116:1633–1641. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikeda RK, Miller M, Nayar J, Walker L, Cho JY, McElwain K, McElwain S, Raz E, Broide DH. J Immunol. 2003;171:4860–4867. doi: 10.4049/jimmunol.171.9.4860. [DOI] [PubMed] [Google Scholar]
- 5.Taube C, Miyahara N, Ott V, Swanson B, Takeda K, Loader J, Shultz LD, Tager AM, Luster AD, Dakhama A, Gelfand EW. J Immunol. 2006;176:3157–3164. doi: 10.4049/jimmunol.176.5.3157. [DOI] [PubMed] [Google Scholar]
- 6.Abonia JP, Hallgren J, Jones T, Shi T, Xu Y, Koni P, Flavell RA, Boyce JA, Austen KF, Gurish MF. Blood. 2006;108:1588–1594. doi: 10.1182/blood-2005-12-012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveira SH, Lukacs NW. Inflamm Res. 2001;50:168–174. doi: 10.1007/s000110050741. [DOI] [PubMed] [Google Scholar]
- 8.Taub D, Dastych J, Inamura N, Upton J, Kelvin D, Metcalfe D, Oppenheim J. J Immunol. 1995;154:2393–2402. [PubMed] [Google Scholar]
- 9.Ochi H, Hirani WM, Yuan Q, Friend DS, Austen KF, Boyce JA. J Exp Med. 1999;190:267–280. doi: 10.1084/jem.190.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abonia JP, Austen KF, Rollins BJ, Joshi SK, Flavell RA, Kuziel WA, Koni PA, Gurish MF. Blood. 2005;105:4308–4313. doi: 10.1182/blood-2004-09-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Sanctis GT, MacLean JA, Qin S, Wolyniec WW, Grasemann H, Yandava CN, Jiao A, Noonan T, Stein-Streilein J, Green FH, Drazen JM. J Clin Invest. 1999;103:507–515. doi: 10.1172/JCI4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moldobaeva A, Wagner EM. Am J Physiol. 2005;288:L1117–L1123. doi: 10.1152/ajplung.00370.2004. [DOI] [PubMed] [Google Scholar]
- 13.Bereta J, Bereta M, Cohen S, Cohen MC. Cell Immunol. 1993;147:313–330. doi: 10.1006/cimm.1993.1072. [DOI] [PubMed] [Google Scholar]
- 14.Reutershan J, Morris MA, Burcin TL, Smith DF, Chang D, Saprito MS, Ley K. J Clin Invest. 2006;116:695–702. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurish MF, Tao H, Abonia JP, Arya A, Friend DS, Parker CM, Austen KF. J Exp Med. 2001;194:1243–1252. doi: 10.1084/jem.194.9.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandrasekar B, Melby PC, Sarau HM, Raveendran M, Perla RP, Marelli-Berg FM, Dulin NO, Singh IS. J Biol Chem. 2003;278:4675–4686. doi: 10.1074/jbc.M207006200. [DOI] [PubMed] [Google Scholar]
- 17.Wong CK, Wang CB, Li ML, Ip WK, Tian YP, Lam CW. Int Immunopharmacol. 2006;6:1859–1871. doi: 10.1016/j.intimp.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Ueno H, Pradhan S, Schlessel D, Hirasawa H, Sumpio BE. Cardiovasc Toxicol. 2006;6:39–50. doi: 10.1385/ct:6:1:39. [DOI] [PubMed] [Google Scholar]
- 19.Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, Moore MW. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 20.Schuh JM, Blease K, Hogaboam CM. J Immunol. 2002;168:1447–1456. doi: 10.4049/jimmunol.168.3.1447. [DOI] [PubMed] [Google Scholar]
- 21.Sue RD, Belperio JA, Burdick MD, Murray LA, Xue YY, Dy MC, Kwon JJ, Keane MP, Strieter RM. J Immunol. 2004;172:3860–3868. doi: 10.4049/jimmunol.172.6.3860. [DOI] [PubMed] [Google Scholar]
- 22.Johnston RA, Mizgerd JP, Shore SA. Am J Physiol. 2005;288:L61–L67. doi: 10.1152/ajplung.00101.2004. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I. J Exp Med. 1994;179:1145–1154. doi: 10.1084/jem.179.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crapper RM, Schrader JW. J Immunol. 1983;131:923–928. [PubMed] [Google Scholar]
- 25.Guy-Grand D, Dy M, Luffau G, Vassalli P. J Exp Med. 1984;160:12–28. doi: 10.1084/jem.160.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arinobu Y, Iwasaki H, Gurish MF, Mizuno S, Shigematsu H, Ozawa H, Tenen DG, Austen KF, Akashi K. Proc Natl Acad Sci USA. 2005;102:18105–18110. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overbergh L, Valckx D, Waer M, Mathieu C. Cytokine. 1999;11:305–312. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]
- 28.Kim DC, Hsu FI, Barrett NA, Friend DS, Grenningloh R, Ho IC, Al Garawi A, Lora JM, Lam BK, Austen KF, Kanaoka Y. J Immunol. 2006;176:4440–4448. doi: 10.4049/jimmunol.176.7.4440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.