Abstract
Class-switch recombination (CSR) enables IgM-producing B cells to switch to the production of IgG, IgE, and IgA. The process requires germ-line (GL) transcription that initiates from promoters upstream of switch (S) sequences and is regulated by the 3′ regulatory region (3′RR) located downstream of the Ig heavy chain (IgH) locus. How the 3′RR effect its long-range activation is presently unclear. We generated a mouse line in which Iγ3 GL promoter was replaced by Iγ1. We found that GL transcription could initiate from the inserted Iγ1 promoter and was induced by increased concentrations of IL-4 and that the transcripts were normally spliced. However, when compared with GL transcripts derived from the endogenous Iγ1 promoter in the same stimulation conditions, those from the inserted Iγ1 promoter were less abundant. CSR to Cγ3 was abrogated both in vivo and in vitro. The results strongly suggest that the endogenous Iγ1 promoter insulates the inserted Iγ1 from the long-range activating effect of the 3′RR. The implications of our findings are discussed in light of the prominent models of long-distance activation in complex loci.
Keywords: 3′ regulatory region, Ig heavy chain locus, promoter competition
Two types of rearrangements take place at the Ig locus: V(D)J assembly that generates the variable (V) region genes at the IgH and IgL loci during early stages of B cell development and class-switch recombination (CSR) at the IgH locus of mature B cells. Another genetic alteration known as somatic hypermutation targets the V exons that acquire point mutations, allowing selection of mutated B cell clones that produce higher-affinity antibodies (1).
In the mouse, the constant (C) region genes are organized in the following order: 5′-Cμ-Cδ-Cγ3-Cγ1-Cγ2b-Cγ2a-Cε-Cα-3′. CSR occurs between highly repetitive switch (S) sequences located upstream of all of the C genes except Cδ. The S sequences differ both in size and in the nature of the repeats (2). CSR is often directed to the same S sequences on both homologous chromosomes (3–5) and is preceded by a biallelic GL transcription directed by I GL promoters (6). The transcripts run through the I exon and the S sequences and undergo polyadenylation downstream of the C exons. Splicing enables fusion of the I exon to the C region and excision of the intervening sequences, yielding sterile transcripts (1). The processing of GL transcripts is required for efficient CSR (7–11). CSR involves several ordered steps that begin with the recognition and targeting of S regions in a GL transcription- and higher-order structures-dependent manner, and the initiation of staggered DNA breaks within partner S sequences by activation-induced cytidine deaminase (AID) (12, 13), a single-stranded-DNA-specific cytidine deaminase (14–17). Studies showed that GL transcription was necessary for the accessibility of S sequences to AID through at least two mechanisms: (i) GL transcripts form RNA-DNA hybrids with the template strand (18–22), whereas the single-stranded nontemplate strand forms long and stable R-loops in vivo, which may serve as substrates for AID (23). The latter was shown to associate with the chromatin of the target S sequences in a GL transcription-dependent manner through a direct interaction with the transcription machinery (24). (ii) AID is phosphorylated in a B cell-specific manner enabling an interaction with replication protein A (25–27).
Activation and targeting of CSR can be mimicked in vitro by a combination of certain mitogens and cytokines to induce or suppress GL transcription of specific C genes (2). Cis-regulatory elements located upstream of the GL promoters or downstream of the IgH locus control CSR by regulating GL transcription (28). Different knockout experiments demonstrated the importance of GL transcription for efficient CSR (29–32) and its regulation by the 3′ regulatory region (3′RR), which comprises four DNase I hypersensitive sites with enhancer activity, hs3a, hs1–2, hs3b, and hs4 (28). Any attempt to tackle the role of the 3′RR in CSR in vivo must accommodate at least three facts : (i) GL transcription is necessary for the accessibility of S sequences to AID; (ii) although necessary, GL transcription is not sufficient and processing of GL transcripts is required for efficient CSR; (iii) GL transcription is regulated in a major part by cis-regulatory elements upstream of the GL promoters or downstream of the IgH locus.
How the 3′RR effects its long-range activation is presently unclear. Essentially four models have been proposed to account for long-distance interactions between enhancers and promoters. In the looping model, a physical interaction is established between the enhancer and the promoter through protein–protein interactions with a looping-out of the intervening sequences. In the scanning model, the enhancer recruits its specific factors and the complex slides along the chromatin fibre culminating in the contact with the factors bound by the target promoter and looping-out of the intervening sequences. The linking model invokes modified chromatin domains that are established between the enhancer and the promoter through a chain of higher-order complexes generated by facilitator proteins. Finally, the enhancer may direct the promoter to subnuclear compartments where high levels of transcription are achieved (33–37).
Here, we replaced Iγ3 GL promoter with Iγ1 and investigated the consequences of the mutation on GL transcription derived from the endogenous and the replacement Iγ1 promoters and on CSR to the corresponding isotypes.
Results
Replacement of Iγ3 GL Promoter by Iγ1.
To generate the mutant mouse line, a 2-kb PmeI-EcoRV fragment containing the Iγ3 promoter region and the proximal part of Iγ3 exon (38, 39) was replaced by an ≈0.5-kb PCR-amplified fragment comprising the Iγ1 enhancer/promoter with known DNA-binding sites for inducible transcription factors (40, 41) [supporting information (SI) Scheme 1]. We chose to remove 2 kb instead of the ≈0.5-kb sequence that contains Iγ3 promoter to get ride of potential unidentified regulatory elements upstream of the Iγ3 promoter. The targeting vector was designed so that the homologous recombination event leads to a chimeric sequence made up of the Iγ1 enhancer/promoter (hereafter the inserted Iγ1 promoter) and of the 368-bp-long distal part of Iγ3 exon with its canonical splice donor site. A neor-specific probe allowed to exclude random integration events in the two recombinant ES clones that were injected into blastocysts (data not shown). Both clones allowed GL transmission of the mutation. The neor gene was deleted by mating homozygous N/N mice (neor-containing alleles) with a Cre-expressing transgenic mice (the homozygous floxed mice will be referred to as Iγ1/Iγ3 mice and the heterozygous mice will be referred to as Δ/+). We amplified the Iγ1/Iγ3 chimera from genomic DNA of mutant mice and checked that no mutation occurred in the inserted sequence (SI Scheme 1).
Analysis of Serum IgG3 in Iγ1/Iγ3 Mice.
To analyze the sera, Iγ1/Iγ3 mice were bred, and the progeny were bled at week 8. ELISA showed a complete absence of IgG3 in the sera of unimmunized Iγ1/Iγ3 mice, whereas IgG3 was readily detected in the sera of WT control mice. The other isotypes tested were found in comparable titers in the sera of WT and Iγ1/Iγ3 mice (SI Fig. 6 A and B). Thus, replacement of Iγ3 promoter by Iγ1 leads to a specific shutdown of IgG3 production in vivo.
IgG3 Production by in Vitro-Activated Splenocytes.
To check that the lack of IgG3 production by homozygous mice is a B cell autonomous process, we resorted to in vitro activation of splenocytes. CSR to IgG1 can be mimicked in vitro by culturing splenic B cells in the presence of anti-CD40+IL4 or LPS+IL4. The treatments activate Iγ1 GL promoter, which contains NF-κB and IL4-responsive motifs (SI Scheme 1), thus enhancing GL transcription from Iγ1 promoter and subsequent switching to IgG1. In the Iγ1/Iγ3 mouse line, the treatment should enable transcription and hence the accessibility of the Sγ1 region downstream of the endogenous Iγ1 promoter and of Sγ3 sequences downstream of the inserted Iγ1 promoter, potentially leading to the production of IgG1 and IgG3, respectively.
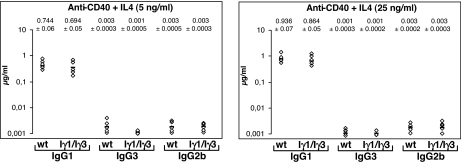
Total splenocytes from littermates were stimulated with anti-CD40+IL4 for 5 days, and supernatants were analyzed by ELISA. At 5 ng/ml of IL4, we found comparable IgG1 secretion in Iγ1/Iγ3 supernatants and WT controls. An increase in IgG1 production was detected for both genotypes by increasing the concentration of IL4 to 25 ng/ml (Fig. 1). In contrast, IgG3 production in Iγ1/Iγ3 supernatants was at the background level at both concentrations of IL4 (Fig. 1). The same pattern was found for IgG3 production when Iγ1/Iγ3 splenocytes were stimulated with LPS+IL4 at both IL4 concentrations (SI Fig. 7). When splenocytes were cultured in the presence of LPS alone, a treatment that induces switching to IgG3 and IgG2b, no IgG3 was detected in the supernatants of Iγ1/Iγ3 splenocytes in contrast to WT and IgG2b controls (SI Fig. 7).
Fig. 1.
Analysis of Ig production in the culture supernatants. ELISA analysis of IgG1, IgG3, and IgG2b secretion after anti-CD40+IL4-stimulation at 5 ng/ml and 25 ng/ml of IL4. Splenocytes from five littermates of WT or Iγ1/Iγ3 mice were analyzed for anti-CD40+IL4-induced IgG1, IgG3, and IgG2b secretion 5 days after stimulation. The experiment was performed twice. Mean Ig levels from two independent experiments and mean deviations are indicated.
Surface Expression on in Vitro-Activated Splenocytes.
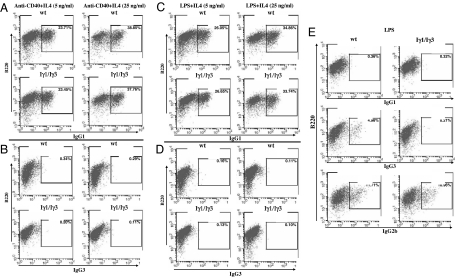
Surface expression of IgG3, IgG1, IgG2b, and IgA on LPS-, anti-CD40+IL4-, LPS+IL4-, or LPS+TGF-β-activated splenocytes was monitored by flow cytometry, using an anti-B220 antibody and anti-IgG3, anti-IgG1, anti-IgG2b, or anti-IgA antibodies. Surface expression of IgG1 was comparable in WT and Iγ1/Iγ3 B220+ splenocytes stimulated with anti-CD40+IL4 and similarly increased with IL4 concentration (≈23% at 5 ng/ml of IL4 and ≈38% at 25 ng/ml of IL4 for both genotypes) (Fig. 2A). Such increase in surface expression was not observed for IgG3, IgG2b, or IgA, which remained at the background level in the same stimulation conditions for both WT and Iγ1/Iγ3 B cells (Fig. 2B and SI Fig. 8). A similar pattern was found with LPS+IL4 stimulation (Fig. 2 C and D and SI Fig. 8). In contrast, LPS stimulation allowed surface expression of IgG2b to comparable levels between WT and Iγ1/Iγ3 B220+ splenocytes but failed to induce surface expression of IgA, the latter being induced by LPS+TGF-β (Fig. 2E and SI Fig. 8). IgG3-surface expression was induced in the WT- but not in the mutant LPS-activated splenocytes (Fig. 2E).
Fig. 2.
Cell surface Ig expression on stimulated splenocytes. (A and B) Splenocytes from WT or Iγ1/Iγ3 mice were cultured for 5 days with anti-CD40+IL4 at 5 or 25 ng/ml of IL4, and stained with anti-B220 and anti-IgG1 (A) or anti-IgG3 (B). (C and D) Splenocytes from WT or Iγ1/Iγ3 mice were cultured for 5 days with LPS+IL4 at 5 or 25 ng/ml of IL4 and stained with anti-B220 and anti-IgG1 (C) or anti-IgG3 (D). (E) Splenocytes from WT or Iγ1/Iγ3 mice were cultured for 5 days with LPS and stained with anti-B220 and anti-IgG1, anti-IgG3, or anti-IgG2b. The percentages of switched splenic B cells among the B220+ populations are indicated. The data shown are representative of two independent experiments.
Thus, increasing IL4 concentration leads to a parallel increase in IgG1 but not in IgG3 surface expression, again indicating a shut-down of IgG3 production in Iγ1/Iγ3 splenocytes. In addition, LPS and LPS+TGF-β stimulations clearly show that the defect does not target downstream isotypes.
Analysis of GL Transcription.
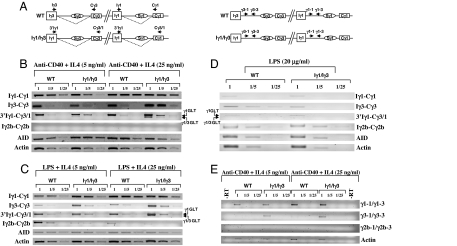
Given the abrogation of IgG3 production in vitro and in vivo and the extinction of IgG3 surface expression on the Iγ1/Iγ3 splenocytes, it was critical to check the GL transcription that initiates from the inserted Iγ1 promoter. Total RNAs from anti-CD40+IL4- or LPS+IL4-activated WT and Iγ1/Iγ3 splenocytes were reverse-transcribed and amplified in semiquantitative conditions, using isotype-specific GL transcript primers. For the inserted Iγ1 promoter, we used a pair of primers specific for the distal part of Iγ3 exon and the Cγ3–1 exon (Iγ3-Cγ3) and a primer in the 3′part of Iγ1 promoter, which should, in combination with a primer whose sequence is common to Cγ3 and Cγ1, amplify both the hybrid γ3 (797 bp) and the native γ1 (900 bp) transcripts (3′Iγ1-Cγ3/1 primers) (Fig. 3A).
Fig. 3.
Analysis of GL transcription. (A) Relative position of some primers used for RT-PCR on the spliced (dotted line) γ3 and γ1 transcripts (Left) and on unspliced transcripts (Right) from WT and Iγ3/Iγ1 alleles respectively (not to scale). (B) RT-PCR was performed on WT or Iγ1/Iγ3 GL transcripts from anti-CD40+IL4-activated splenocytes RNA (day 3) for Iγ1-Cγ1, Iγ3-Cγ3, Iγ2b-Cγ2b, 3′Iγ1-Cγ3/1 GL, or AID and actin transcripts, respectively. Single-stranded cDNAs or dilutions thereof (1/5 and 1/25) were subjected to PCR, using appropriate primers. Arrows indicate transcripts initiating from the endogenous Iγ1 promoter (upper band, referred to as γ1 GLT) or from the replacement promoter (lower band, γ1/γ3 GLT). (C) RT-PCR was performed as in A on GL transcripts from LPS+IL4-activated splenocytes. (D) RT-PCR was performed as in A on GL transcripts from LPS-activated splenocytes. (E) RT-PCR was performed on WT or Iγ1/Iγ3 RNAs from nuclei of anti-CD40+IL4-activated splenocytes for γ1, γ3, γ2b, or β-actin unspliced transcripts, respectively. Single-stranded cDNAs or dilutions thereof (1/5 and 1/25) were subjected to PCR, using appropriate primers.
By using isotype-specific primers, we noted an increase in the abundance of the Iγ1-Cγ1 transcripts for both genotypes by increasing the concentration of IL4 to 25 ng/ml. A different picture emerged for Iγ3-Cγ3 transcripts, where the increase in IL4 led to more abundant transcripts only in Iγ1/Iγ3 splenocytes (Fig. 3B). Sequencing of the cDNA showed normal splicing to Cγ3 (SI Fig. 9). Essentially the same pattern was found in LPS+IL4-activated splenocytes (Fig. 3C). Interestingly, by using the 3′Iγ1-Cγ3/1 primers, we detected both the native γ1 and the hybrid γ3 transcripts. More importantly, the abundance of the transcripts mirrored that seen with isotype-specific primers (Iγ3-Cγ3 and Iγ1-Cγ1) (Fig. 3 B and C). Although some Iγ2b-Cγ2b transcripts were detected in LPS+IL4 stimulation, they were efficiently suppressed by increasing IL4 concentration, and no Iγ2b-Cγ2b transcripts were detected in anti-CD40+IL4 stimulation (Fig. 3 B and C). In contrast, upon LPS stimulation, Iγ2b-Cγ2b transcripts were equally abundant in WT and Iγ1/Iγ3 splenocytes. Iγ3-Cγ3 transcripts were also readily detected in WT splenocytes but were much less abundant in Iγ1/Iγ3 splenocytes. The use of the 3′Iγ1–Cγ3/1 pair allowed some amplification of both the native γ1 and the hybrid γ3 transcripts (Fig. 4D). No difference between WT and Iγ1/Iγ3 splenocytes was found for AID transcripts in all stimulation conditions tested, but we noticed that AID transcripts were more abundant upon stimulation with anti-CD40+IL4 than with LPS+IL4 or LPS alone.
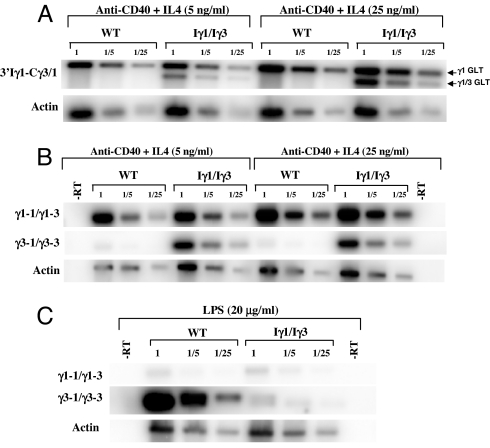
Fig. 4.
Quantification of GL transcripts. (A) Total RNA (day 3) from anti-CD40+IL4-activated WT or Iγ1/Iγ3 splenocytes was reverse-transcribed and the corresponding single-stranded cDNAs or dilutions thereof (1/5 and 1/25) were subjected to PCR, using 3′Iγ1-Cγ3/1 or actin primers. For quantification of the signals, a Southern blot analysis was performed. The nylon membranes were hybridized to 5′ end-radiolabeled Cγ3/1 or actin probes. Hybridization signals were quantified by a PhosphorImager. The ratios of 3′Iγ1-Cγ3/1 signals were corrected to the corresponding actin ratios. Arrows indicate transcripts initiating from the endogenous Iγ1 promoter (upper band, γ1 GLT) or from the replacement promoter (lower band, γ1/γ3 GLT). (B) For quantification of unspliced transcripts, nuclear RNA from of anti-CD40+IL4-activated WT or Iγ1/Iγ3 splenocytes was reverse-transcribed, and the corresponding single-stranded cDNAs or dilutions thereof (1/5 and 1/25) were subjected to PCR, using intronic primer pairs. For the signals' quantification, a Southern blot analysis was performed. The nylon membranes were hybridized to randomly primed intronic probes. Hybridization signals were quantified and corrected as in A. (C) Unspliced transcripts from LPS-activated WT or Iγ1/Iγ3 splenocytes were reverse-transcribed, and the corresponding single-stranded cDNAs or dilutions thereof (1/5 and 1/25) were subjected to PCR, using intronic primer pairs. The signals' quantification was performed as in A.
To further check that transcription of Sγ3 does occur in Iγ1/Iγ3 splenocytes, we designed a set of primers that specifically amplify unspliced transcripts. With the primers specific for the γ1 intronic sequence, an amplification was found for both genotypes and correlated with IL4 concentration. A faint signal was detected with γ3 intronic primers in WT nuclei. In contrast, more amplification was observed in Iγ1/Iγ3 nuclei, but the correlation between the intensity of the signals and the increase of IL4 concentration was not obvious. In contrast, no γ2b intronic sequences could be amplified at detectable level in both stimulation conditions (Fig. 3E).
Quantification of GL Transcripts.
The use of 3′Iγ1-Cγ3/1 primers allowed amplification of both the native γ1 and the hybrid γ3 spliced GL transcripts. This offered a unique opportunity to quantify the two transcript species from the same amplification reaction. Quantification of the transcripts showed a 2- to 3-fold induction of the native γ1 transcripts by increasing IL4 concentration for both WT and mutant genotypes (Fig. 4A and SI Fig. 10). The native γ1 transcripts were 10–12 times more abundant than the chimeric γ3 transcripts at 5 ng/ml of IL4. Increasing IL4 concentration to 25 ng/ml led to a parallel induction of both species, yet the abundance of the chimeric γ3 transcript was always inferior to that of the native γ1 transcript (4–6 times less) (Fig. 4A and SI Fig. 10). Intriguingly, comparison of the chimeric γ3 transcript at 25 ng/ml (a concentration at which no switching to Cγ3 was detected) with the native γ1 at 5 ng/ml (at which a substantial switching to Cγ1 occurred, but none to Cγ3) showed only 2–4 times fewer γ3 transcripts (see Discussion).
Induction of GL transcription was also quantified for unspliced transcripts. Increasing IL4 concentration led to a 2- to 4-fold increase in the abundance of γ1 transcripts for WT and mutant genotypes, the increase was less marked for γ3 transcripts (≈1.5-fold) (Fig. 4B and SI Fig. 10). Upon LPS stimulation, γ1 unspliced transcripts from both WT and Iγ1/Iγ3 nuclei yielded faint but equal signals. In contrast, γ3 unspliced transcripts were far more abundant in WT than in Iγ1/Iγ3 nuclei (an ≈40-fold increase) (Fig. 4C and SI Fig. 10).
We conclude that GL transcription can initiate from the inserted Iγ1 promoter but the abundance of the chimeric γ3 transcripts could not reach that of the native γ1 transcripts at any of the stimulation conditions tested.
Discussion
We generated a mouse line in which Iγ3 GL promoter was replaced by Iγ1 leading to a duplication of Iγ1 GL promoter at the IgH locus. Both Iγ1 promoters should in principle respond to the same extracellular stimulus, have the same strength, be under the control of the same known cis-regulatory elements, and recruit the same set of specific transcription factors. Although we cannot formally exclude the possibility that the inserted Iγ1 sequence lacks some unidentified activating elements, we think it unlikely to lie solely at the basis of our findings because of the abundant GL transcription that we detect by increasing IL4 concentration. This observation indicates that the inserted sequence responds to the synergistic effect of anti-CD40 and IL4 (or LPS+IL4).
We found that GL transcription could initiate from the inserted Iγ1 promoter after appropriate stimulation and the transcripts were normally spliced to Cγ3. Although we did not map precisely the transcription initiation sites within the inserted Iγ1 promoter region, the RT-PCR results on both spliced and unspliced transcripts clearly showed that the targeted Sγ3 region was transcribed in an IL4-dependent manner. However, when compared with GL transcription from the endogenous Iγ1 promoter, the inserted Iγ1 promoter was less abundant at 25 ng/ml of IL4 and much less abundant at 5 ng/ml of IL4.
Why is GL transcription from the inserted Iγ1 promoter decreased? Beside the potential lack of unidentified transcriptional elements, we cannot rule out the possibility that the chimeric γ1/γ3 GL transcript is less stable than the native γ1 GL transcript. Another possibility could be a promoter occlusion mechanism associated with transcriptional read-through past the polyadenylation site and by which transcription of the upstream gene disrupts that of the downstream gene. At the mouse β-globin locus, which shares several similarities with the IgH locus, such a mechanism has recently been proposed to explain the activation of the βh0 promoter upon deletion of the upstream Ey promoter (42). This mechanism cannot account for our finding, because a large distance separates the Iγ1 promoters, and, more importantly, because one would then expect a decrease of GL transcription from the endogenous Iγ1 promoter.
A more plausible explanation is that Iγ1 promoters compete for the 3′RR, which would provide the rate-limiting activity (43, 44). In light of the current models of long-distance interactions, the looping model would predict that the 3′RR would interact with the upstream promoter with probability equal to that of the downstream promoter but with one promoter at the time. In addition, these interactions should take place on both chromosomes given the biallelic nature of GL transcription (6). This should lead to an equivalent abundance of GL transcripts derived from both the replacement and the native Iγ1 promoters, because they presumably have the same strength, recruit the same transcription factors, and respond to the same signal. We do not detect equal abundance of GL transcripts from Iγ1 promoters, therefore the looping model in its simplest form is unlikely to account for our findings unless additional facilitating mechanisms are invoked, such as the physical arrangement of the promoters and their distance from the 3′RR. Two other lines of evidence indirectly argue against the looping model: (i) the enhancer blocking activity of insulators when they are inserted between a promoter and an enhancer (33, 34, 37) and (ii) the inhibiting effect of the neor gene on GL transcription. In mutant mice in which the neor gene was inserted at different sites of the IgH constant locus, GL transcription initiated from the exogenous phosphoglycerate kinase or thymidine kinase promoters, leading to substantial switching to the targeted regions. The critical observation was that GL transcription (and conversely CSR to the corresponding genes) was impaired from upstream but not from downstream GL promoters relative to the insertion site of the selectable marker, with the seemingly exception of Cγ1 (discussed in refs. 11 and 45). Studies have reported that transcription from Iγ1 promoter is the less affected by 3′RR mutations (44, 46). However, this mild phenotype may simply reflect the redundancy among 3′RR enhancers and the availability of an upstream Iγ1 enhancer (41). A deletion encompassing the whole 3′RR would have a more severe impact on γ1 expression (47), although, in the latter case, effects resulting from the ectopic insertion of the transgenes cannot be ruled out.
Our results do not disprove the subnuclear redirection model (see below); however, it is difficult to figure out why the 3′RR should direct the endogenous Iγ1 but not the inserted Iγ1 promoter to the transcription factories (48). Although the large distance between the Iγ1 promoters may be invoked to explain their different outcomes, the phenotype of mice bearing insertions of the neor gene at the IgH constant locus suggests that the distance between the promoters might not be the real issue, because transcription from upstream promoters was severely impaired regardless of their distance from the neor gene.
Our data support the notion that an activation signal originating from the 3′RR is interrupted by the active endogenous Iγ1 promoter and hence does not reach the inserted Iγ1 promoter. Although our current data do not argue against the linking model, we favor the view that the active endogenous Iγ1 promoter somehow insulates the upstream Iγ1 promoter from the activating effect of the 3′RR, perhaps through a tracking/scanning model. Based on the model of West and Fraser (37), one possibility would be that, in response to the appropriate stimulation, the 3′RR directs the IgH constant locus to a transcription factory where some opening of the constant locus occurs. The signal-mediated selection of Iγ1 GL promoters would allow recruitment of specific factors that bind to proximal sequences, enabling a 3′RR-independent transcription to occur. The 3′RR-transcription factors complex would slide along the chromatin fiber; alternatively, the latter is reeled in until a stable contact is established between the 3′RR and the endogenous Iγ1 promoter, allowing a high level of transcription. The “sequestration” of the 3′RR by the endogenous Iγ1 promoter would hamper interaction between the 3′RR and the upstream Iγ1 promoter. Transcription activation from the upstream promoter would thus rely mainly on proximal sequences that seem insufficient for CSR. This model would predict that insertion of Iγ1 promoter downstream of the endogenous Iγ1 promoter would lead to a decrease of GL transcription derived from the endogenous promoter. This hypothesis is currently being tested.
An important finding in this study is the complete and specific inhibition of CSR to Cγ3 in Iγ1/Iγ3 mice both in vivo and in vitro. Given the requirement of GL transcription for CSR, one potential explanation is that, despite induced GL transcription from the inserted Iγ1 promoter, the minimal threshold of GL transcription required for detectable CSR has not been reached. In this scenario, by sequestering the 3′RR, the endogenous Iγ1 promoter would transcriptionally out-compete the inserted Iγ1 promoter leading to CSR to IgG1 but not to IgG3. Alternatively, one might speculate that the severity of the inhibition is such that it cannot be explained solely on the basis of lesser GL transcription from the inserted Iγ1 promoter and that insulation of the 3′RR may have profound effects on CSR to Sγ3 in a more complex way than by simply impairing its GL transcription. In this scenario, although decreased, GL transcription derived from the inserted Iγ1 promoter would be sufficient to ensure some CSR to Cγ3; however, retention of the 3′RR by the downstream promoter may somehow block the recombination step at Sγ3. An intriguing possibility would be that, in addition to its role as a transcriptional control element, the 3′RR may act as a switch recombination enhancer that might play a role in conferring isotype specificity. How the 3′RR achieves this function is presently unclear, a situation that is reminiscent of Eμ enhancer, which acts both as a transcriptional and a recombinational control element during V(D)J recombination (49, 50).
Materials and Methods
Targeting Vector and Mice.
The Iγ1-targeting construct was generated by using a plasmid containing an ≈8-kb XhoI-BamHI fragment spanning Iγ3 and Sγ3. A ClaI linker was inserted in PmeI and EcoRV sites and the resulting plasmid was digested with ClaI and self-ligated. The Iγ1 promoter was PCR-amplified by using ZOγ1–1 and ZOγ1–2 primers. The PCR product was checked by sequencing and inserted downstream of the neor gene. The whole cassette was then excised as a ClaI fragment and inserted in ClaI site of the targeting construct. An HSV tk gene was inserted in the NotI site for negative selection. The ES cell line CK35 [kindly provided by C. Kress (Institut Pasteur, Paris, France)] was transfected by electroporation and selected by using G418 (300 μg/ml) and gancyclovir (2 μM). Recombinant clones were identified by Southern blot analysis after an EcoRI digest with external probes: a 1.0-kb EcoRI-XhoI fragment as a 5′ probe and a 1.7-kb BamHI-SphI as a 3′ probe. Two ES clones showing homologous recombination were injected into C57BL/6 blastocysts and the male chimeras were then mated with C57BL/6 females. GL transmission of the mutation was checked by Southern blot, using the same digest and probes. Homozygous N/N mutant mice were mated with EIIa-cre transgenic mice [a kind gift of H. Westphal (National Institute of Child Health and Human Development, Bethesda, MD), used under a noncommercial research license agreement from Dupont Pharma]. The progeny was checked by Southern blot for Cre-mediated deletion, using a Δ probe and a 440-bp PCR-amplified fragment, using Iγ3f and γ3–4 primers. The fragment spans the 3′ part of Iγ3 exon and the adjacent downstream intronic sequences. The experiments on mice have been carried out according to the Centre National de la Recherche Scientifique Ethical Committee guidelines and approved by the Committee.
Spleen Cell Cultures.
Single-cell suspensions of splenocytes from 6- to 8-week-old mice were activated in vitro at a density of 106 cells per ml in RPMI medium 1640 supplemented with 10% FCS, 50 μM 2-ME, and 20 μg/ml of LPS (S. typhimurium; Sigma) or 500 ng/ml of anti-CD40 (R&D Systems). IL4 (R&D Systems) was added at 5 ng/ml or at 25 ng/ml. TGF-β (R&D Systems) was added at 1 ng/ml. At day 3, aliquots of cells were removed for RNA preparation.
Flow Cytometry Analysis.
At day 5 after stimulation, splenocytes (5 × 105 cells per assay) were labeled by using spectral red-conjugated anti-B220 and FITC-conjugated anti-IgG3, anti-IgG2b, anti-IgG1, or anti-IgA (BD PharMingen). Isotype controls were included in each experiment for each stimulation condition. Data were obtained on 1.5 × 104 viable cells by using a Beckman Coulter XL apparatus.
ELISAs.
Sera or supernatants from spleen cell cultures (harvested at day 5 after stimulation) were analyzed for the presence of IgM, IgG3, IgG1, IgG2a, IgG2b, and IgA by ELISA as described in ref. 46. Serum analysis was performed on the progeny of two independent breedings. For each progeny, six mice per genotype were independently analyzed twice.
Oligonucleotides and RT-PCR Analysis of GL Transcription.
The oligonucleotides are listed in SI Fig. 10. The RT-PCR conditions for spliced transcripts and the expected sizes of the PCR products have been described (46, 51). After agarose gel electrophoresis, the PCR products were transferred to nylon membranes (Perkin–Elmer) and hybridized to 5′ end-radiolabeled Cγ3/1 or mActi-5 oligonucleotides. The hybridization signals were quantified by a phosphorImager (Molecular Dynamics). To avoid potential PCR saturation problems with undiluted samples, the comparisons were based on the diluted samples' signals and corrected to the corresponding actin signals.
For unspliced transcripts, splenic B cells from 6-week-old mice were purified as the negative fraction, using anti-CD43 beads and LS columns (Miltenyi) according to the supplier's protocol. B cells were stimulated with LPS (20 μg/ml) or anti-CD40 (500 ng/ml) and IL4 (at 5 or 25 ng/ml) at a density of 106 cells per ml. After 48 h, the cells were washed twice with ice-cold PBS, gently resuspended in 10 mM Tris (pH7.4), 10 mM NaCl, 2.5 mM MgCl2, and 0.5% Nonidet P-40 and left on ice for 5 min. The cell lysates were carefully added on the top of an equal volume of 10 mM Tris (pH7.4), 10 mM NaCl, 2.5 mM MgCl2, 10% sucrose, and 0.5% Nonidet P-40 and centrifuged for 10 sec at 4,800 rpm (A4-44 rotor; Eppendorf). The nuclear pellets were lysed in TRIzol (Invitrogen), and RNA was purified according to the supplier's instructions. The contaminating DNA was removed by adding DNase I (Invitrogen), the purified RNA was reverse-transcribed following the manufacturer's protocol, and the resulting single-strand cDNA was subjected to PCR, using γ1–1/γ1–3, γ3–1/γ3–3, or Acti6/Acti7 primer pairs. All of the expected PCR products are 246 bp long, and the corresponding sequences are located upstream of the S sequences (Sγ3, Sγ1, and Sγ2b) and within the first intron of the β-actin gene, respectively. PCR controls included RNA substrates without reverse transcription and water with the primers. PCR conditions were as follows: 94°C for 5 min, 35 cycles (94°C for 2 min, 55°C for 30 sec, and 72°C for 1 min). The RT-PCR products were transferred to nylon membranes and hybridized to the corresponding amplicons that were radiolabeled by random-priming. The hybridization signals were quantified as described above.
Supplementary Material
ACKNOWLEDGMENTS.
We thank M. S. Neuberger, J.-C. Weill, and C.-A. Reynaud, for critical reading of the manuscript; Michel Cogné for warm support; and S. Aoufouchi and Frederic Delbos for much help. We thank the reviewers for very helpful comments. This work was supported by fellowships from the Ministère de la Recherche et de l'Education Nationale, the Fondation pour la Recherche Médicale, and European Molecular Biology Organization-Short-Term Fellowship (Z.O.); Association pour la Recherche sur la Cancer Grant 3789/7946; Ligue Conte le Cancer (Comité Tarn et Garonne); and Cancéropôle Grand Sud Ouest: L'instabilité génétique comme signature péjorative de la maladie (ACI 2005–2007).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0608364104/DC1.
References
- 1.Chaudhuri J, Alt FW. Nat Rev Immunol. 2004;4:541–552. doi: 10.1038/nri1395. [DOI] [PubMed] [Google Scholar]
- 2.Stavnezer J. Curr Top Microbiol Immunol. 2000;245:127–168. doi: 10.1007/978-3-642-59641-4_6. [DOI] [PubMed] [Google Scholar]
- 3.Radbruch A, Muller W, Rajewsky K. Proc Natl Acad Sci USA. 1986;83:3954–3957. doi: 10.1073/pnas.83.11.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hummel M, Berry JK, Dunnick W. J Immunol. 1987;138:3539–3548. [PubMed] [Google Scholar]
- 5.Winter E, Krawinkel U, Radbruch A. EMBO J. 1987;6:1663–1671. doi: 10.1002/j.1460-2075.1987.tb02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delpy L, Le Bert M, Cogné M, Khamlichi AA. Eur J Immunol. 2003;33:2108–2113. doi: 10.1002/eji.200323969. [DOI] [PubMed] [Google Scholar]
- 7.Lorenz M, Jung S, Radbruch A. Science. 1995;267:1825–1828. doi: 10.1126/science.7892607. [DOI] [PubMed] [Google Scholar]
- 8.Hein K, Lorenz MG, Siebenkotten G, Petry K, Christine R, Radbruch A. J Exp Med. 1998;188:2369–2374. doi: 10.1084/jem.188.12.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu G, Harriman GR, Stavnezer J. Int Immunol. 1999;11:37–46. doi: 10.1093/intimm/11.1.37. [DOI] [PubMed] [Google Scholar]
- 10.Kuzin II, Ugine GD, Wu D, Young F, Chen J, Bottaro A. J Immunol. 2000;164:1451–1457. doi: 10.4049/jimmunol.164.3.1451. [DOI] [PubMed] [Google Scholar]
- 11.Samara M, Oruc Z, Dougier HL, Essawi T, Cogné M, Khamlichi AA. Int Immunol. 2006;18:581–589. doi: 10.1093/intimm/dxh400. [DOI] [PubMed] [Google Scholar]
- 12.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 13.Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, et al. Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhuri J, Tian M, Khuong C, Chua K, Pinaud E, Alt FW. Nature. 2003;422:726–730. doi: 10.1038/nature01574. [DOI] [PubMed] [Google Scholar]
- 15.Dickerson SK, Market E, Besmer E, Papavasiliou FN. J Exp Med. 2003;197:1291–1296. doi: 10.1084/jem.20030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pham P, Bransteitter R, Petruska J, Goodman MF. Nature. 2003;424:103–107. doi: 10.1038/nature01760. [DOI] [PubMed] [Google Scholar]
- 17.Ramiro AR, Stavropoulos P, Jankovic M, Nussenzweig MC. Nat Immunol. 2003;4:452–456. doi: 10.1038/ni920. [DOI] [PubMed] [Google Scholar]
- 18.Reaban ME, Griffin JA. Nature. 1990;348:342–344. doi: 10.1038/348342a0. [DOI] [PubMed] [Google Scholar]
- 19.Reaban ME, Lebowitz J, Griffin JA. J Biol Chem. 1994;269:21850–21857. [PubMed] [Google Scholar]
- 20.Daniels GA, Lieber MR. Nucleic Acids Res. 1995;23:5006–5011. doi: 10.1093/nar/23.24.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian M, Alt FW. J Biol Chem. 2000;275:24163–24172. doi: 10.1074/jbc.M003343200. [DOI] [PubMed] [Google Scholar]
- 22.Mizuta R, Iwai K, Shigeno M, Mizuta M, Uemura T, Ushiki T, Kitamura D. J Biol Chem. 2003;278:4431–4434. doi: 10.1074/jbc.M209262200. [DOI] [PubMed] [Google Scholar]
- 23.Yu K, Chedin F, Hsieh CL, Wilson TE, Lieber MR. Nat Immunol. 2003;4:442–451. doi: 10.1038/ni919. [DOI] [PubMed] [Google Scholar]
- 24.Nambu Y, Sugai M, Gonda H, Lee CG, Katakai T, Agata Y, Yokota Y, Shimizu A. Science. 2003;302:2137–2140. doi: 10.1126/science.1092481. [DOI] [PubMed] [Google Scholar]
- 25.Basu U, Chaudhuri J, Alpert C, Dutt S, Ranganath S, Li G, Schrum JP, Manis JP, Alt FW. Nature. 2005;438:508–511. doi: 10.1038/nature04255. [DOI] [PubMed] [Google Scholar]
- 26.Pasqualucci L, Kitaura Y, Gu H, Dalla-Favera R. Proc Natl Acad Sci USA. 2006;103:395–400. doi: 10.1073/pnas.0509969103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaudhuri J, Khuong C, Alt FW. Nature. 2004;430:992–998. doi: 10.1038/nature02821. [DOI] [PubMed] [Google Scholar]
- 28.Khamlichi AA, Pinaud E, Decourt C, Chauveau C, Cogné M. Adv Immunol. 2000;75:317–345. doi: 10.1016/s0065-2776(00)75008-5. [DOI] [PubMed] [Google Scholar]
- 29.Jung S, Rajewsky K, Radbruch A. Science. 1993;259:984–987. doi: 10.1126/science.8438159. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Bottaro A, Li SC, Stewart V, Alt FW. EMBO J. 1993;12:3529–3537. doi: 10.1002/j.1460-2075.1993.tb06027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bottaro A, Lansford R, Xu L, Zhang J, Rothman P, Alt FW. EMBO J. 1994;13:665–674. doi: 10.1002/j.1460-2075.1994.tb06305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harriman GR, Bradley A, Das S, Rogers-Fani P, Davis AC. J Clin Invest. 1996;97:477–485. doi: 10.1172/JCI118438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blackwood EM, Kadonaga JT. Science. 1998;281:61–63. doi: 10.1126/science.281.5373.60. [DOI] [PubMed] [Google Scholar]
- 34.Bulger M, Groudine M. Genes Dev. 1999;13:2465–2477. doi: 10.1101/gad.13.19.2465. [DOI] [PubMed] [Google Scholar]
- 35.Engel JD, Tanimoto K. Cell. 2000;100:499–502. doi: 10.1016/s0092-8674(00)80686-8. [DOI] [PubMed] [Google Scholar]
- 36.Francastel C, Schubeler D, Martin DI, Groudine M. Nat Rev Mol Cell Biol. 2000;1:137–143. doi: 10.1038/35040083. [DOI] [PubMed] [Google Scholar]
- 37.West AG, Fraser P. Hum Mol Genet. 2005;14:R101–111. doi: 10.1093/hmg/ddi104. [DOI] [PubMed] [Google Scholar]
- 38.Rothman P, Lutzker S, Gorham B, Stewart V, Coffman R, Alt FW. Int Immunol. 1990;2:621–627. doi: 10.1093/intimm/2.7.621. [DOI] [PubMed] [Google Scholar]
- 39.Gerondakis S, Gaff C, Goodman DJ, Grumont RJ. Immunogenetics. 1991;34:392–400. doi: 10.1007/BF01787490. [DOI] [PubMed] [Google Scholar]
- 40.Lin SC, Stavnezer J. Mol Cell Biol. 1996;16:4591–4603. doi: 10.1128/mcb.16.9.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu MZ, Stavnezer J. EMBO J. 1992;11:145–155. doi: 10.1002/j.1460-2075.1992.tb05037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu X, Eszterhas S, Pallazzi N, Bouhassira EE, Fields J, Tanabe O, Gerber SA, Bulger M, Engel JD, Groudine M, Fiering S. Blood. 2007;109:2210–2216. doi: 10.1182/blood-2006-06-029868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cogné M, Lansford R, Bottaro A, Zhang J, Gorman J, Young F, Cheng HL, Alt FW. Cell. 1994;77:737–747. doi: 10.1016/0092-8674(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 44.Manis JP, van der Stoep N, Tian M, Ferrini R, Davidson L, Bottaro A, Alt FW. J Exp Med. 1998;188:1421–1431. doi: 10.1084/jem.188.8.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seidl KJ, Manis JP, Bottaro A, Zhang J, Davidson L, Kisselgof A, Oettgen H, Alt FW. Proc Natl Acad Sci USA. 1999;96:3000–3005. doi: 10.1073/pnas.96.6.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinaud E, Khamlichi AA, Le Morvan C, Drouet M, Nalesso V, Le Bert M, Cogne M. Immunity. 2001;15:187–199. doi: 10.1016/s1074-7613(01)00181-9. [DOI] [PubMed] [Google Scholar]
- 47.Dunnick WA, Shi J, Graves KA, Collins JT. J Exp Med. 2005;201:1459–1466. doi: 10.1084/jem.20041988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osborne CS, Chakalova L, Brown KE, Carter D, Horton A, Debrand E, Goyenechea B, Mitchell JA, Lopes S, Reik W, Fraser P. Nat Genet. 2004;36:1065–1071. doi: 10.1038/ng1423. [DOI] [PubMed] [Google Scholar]
- 49.Hesslein DG, Schatz DG. Adv Immunol. 2001;78:169–232. doi: 10.1016/s0065-2776(01)78004-2. [DOI] [PubMed] [Google Scholar]
- 50.Jung D, Giallourakis C, Mostoslavsky R, Alt FW. Annu Rev Immunol. 2006;24:541–570. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- 51.Khamlichi AA, Glaudet F, Oruc Z, Denis V, Le Bert M, Cogne M. Blood. 2004;103:3828–3836. doi: 10.1182/blood-2003-10-3470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.