Abstract
Insulin resistance and metabolic syndrome are rapidly expanding public health problems. Acting through the PI3K/Akt pathway, insulin and insulin-like growth factor-1 (IGF-1) inactivate FoxO transcription factors, a class of highly conserved proteins important in numerous physiological functions. However, even as FoxO is a downstream target of insulin, FoxO factors also control upstream signaling elements governing insulin sensitivity and glucose metabolism. Here, we report that sustained activation of either FoxO1 or FoxO3 in cardiac myocytes increases basal levels of Akt phosphorylation and kinase activity. FoxO-activated Akt directly interacts with and phosphorylates FoxO, providing feedback inhibition. We reported previously that FoxO factors attenuate cardiomyocyte calcineurin (PP2B) activity. We now show that calcineurin forms a complex with Akt and inhibition of calcineurin enhances Akt phosphorylation. In addition, FoxO activity suppresses protein phosphatase 2A (PP2A) and disrupts Akt-PP2A and Akt–calcineurin interactions. Repression of Akt–PP2A/B interactions and phosphatase activities contributes, at least in part, to FoxO-dependent increases in Akt phosphorylation and kinase activity. Resveratrol, an activator of Sirt1, increases the transcriptional activity of FoxO1 and triggers Akt phosphorylation in heart. Importantly, FoxO-mediated increases in Akt activity diminish insulin signaling, as manifested by reduced Akt phosphorylation, reduced membrane translocation of Glut4, and decreased insulin-triggered glucose uptake. Also, inactivation of the gene coding for FoxO3 enhances insulin-dependent Akt phosphorylation. Taken together, this study demonstrates that changes in FoxO activity have a dose-responsive repressive effect on insulin signaling in cardiomyocytes through inhibition of protein phosphatases, which leads to altered Akt activation, reduced insulin sensitivity, and impaired glucose metabolism.
Keywords: cardiomyocyte, calcineurin, insulin resistance, cardiomyopathy
Cardiovascular disease is the primary cause of death in patients with obesity and type 2 diabetes mellitus. Insulin resistance, a major mechanism underlying these disorders, also contributes to several cardiovascular disease-promoting factors, which, together, comprise the metabolic syndrome (1). In addition to its deleterious effects on metabolic parameters, such as lipid dyscrasias and blood pressure, insulin resistance of myocardial muscle itself has been documented in patients with type 2 diabetes and in nondiabetic subjects with angiographically proven coronary artery disease (2). Indeed, strong evidence suggests that diabetes affects cardiac structure and function, independent of blood pressure and coronary artery disease (3, 4). Insulin resistance has also been documented in heart tissue undergoing hypertrophic or atrophic remodeling (5, 6). Although much is known already regarding metabolic and insulin signaling pathways in the heart (3, 6, 7), there is continued interest in defining mechanisms that govern insulin resistance and myocardial plasticity.
The O subfamily of Forkhead/winged helix transcription factors (FoxO) plays important roles in regulating cardiac and skeletal muscle remodeling (8–11). FoxO proteins are phylogenetically conserved and regulate key physiological functions, including cell proliferation, cell differentiation, and survival (12–15). In addition, FoxO functions in complex ways to regulate insulin signaling and glucose and lipid metabolism (13, 16). In Drosophila, activation of dFoxO in fat body represses insulin-dependent signaling and increases life span (17). Similarly in mammals, FoxO1 regulates multiple metabolic pathways in liver and pancreatic β-cells (18), and transgenic expression of FoxO1 in various tissues leads to impaired insulin sensitivity and glucose intolerance (18–20). On the other hand, both dFoxO and the mammalian equivalent FoxO1 activate transcription of the insulin receptor and insulin receptor substrate-2 (IRS-2) in Drosophila S2 cells and mouse C2C12 cells, which sensitizes the cellular response to insulin (21). Also, whereas transgenic overexpression of constitutively active FoxO1 (caFoxO1) in liver triggers impaired fasting glucose and hyperinsulinemia (18, 22), acute overexpression of caFoxO1 in liver reduces plasma insulin, glucose, and triglycerides (23). And paradoxically, FoxO3-deficient mice exhibit impaired insulin sensitivity (24). Thus, many questions remain regarding the roles of FoxO proteins in insulin signaling and their potential contribution to myocardial insulin resistance.
An additional layer of complexity exists in that the transcriptional activity of FoxO is regulated by insulin through the phosphoinositide-3 kinase (PI3K)/Akt signaling pathway. Both insulin and insulin-like growth factor-1 (IGF-1) induce PI3K/Akt-dependent phosphorylation of FoxO, which facilitates its interaction with 14-3-3 protein, leading to nuclear exclusion and eventual ubiquitylation-dependent proteasomal degradation (25). Thus, it is well established that Akt (also known as protein kinase B, or PKB) plays a key role in repressing FoxO transcriptional activity.
Immediately upstream of FoxO, the activity of Akt itself is governed by several protein kinases and phosphatases. Akt is activated by phosphorylation at Thr-308 within its catalytic domain by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and by phosphorylation at Ser-473 within a C-terminal hydrophobic motif by mammalian target of rapamycin (mTOR) (26, 27). Recent studies show that phospho-Thr-308 and phospho-Ser-273 are dephosphorylated by protein phosphatase 1 (PP1), protein phosphatase 2A (PP2A), and pleckstrin homology (PH) domain leucine-rich repeat protein phosphatase (PHLPP), a member of the protein phosphatase 2C family (28, 29). Akt regulates a variety of key physiological functions, and there is strong evidence suggesting that defective Akt signaling contributes to development of insulin resistance (30).
Thus, although it is clear that FoxO governs multiple events in the insulin signaling cascade, mediating both positive and negative effects, underlying molecular mechanisms are unknown. Here, we report that sustained activation of FoxO in cardiomyocytes leads to increased Akt phosphorylation and kinase activity. These changes are mediated, at least in part, by decreased activities of the protein phosphatases calcineurin and PP2A and impaired interactions between these proteins and Akt. Furthermore, FoxO-induced increases in Akt activity attenuate the cellular response to insulin in cardiomyocytes and results in decreased glucose uptake. Together, these findings provide a mechanism whereby FoxO activity contributes to reduced insulin signaling in heart.
Results
FoxO Enhances Akt Activity.
FoxO confers both positive and negative feedback in the insulin/Akt signaling cascade. This fact led us to study effects of FoxO on Akt, its proximal upstream regulator. We observed that forced expression in cardiomyocytes of wild-type or a constitutively active (ca) mutant FoxO led to increased phosphorylation of Akt at both Thr-308 and Ser-473 without affecting Akt protein abundance (Fig. 1 A and B). Similar effects were also observed with overexpression of FoxO3 (Fig. 1C), the other major FoxO isoform in heart.
Fig. 1.
FoxO proteins increase basal levels of Akt phosphorylation and kinase activity. Cardiomyocytes were infected with adenovirus encoding GFP, FoxO1-GFP, caFoxO1-GFP, or caFoxO3-GFP, and whole cell lysates were harvested 24 h after infection. (A–C) Western blots (A and C) and densitometric analyses (B) of phospho-Akt (Ser-473 or Thr-308). Results are graphed as mean ± SEM from at least three independent experiments with duplicate or triplicate samples. (D) Western blot analyses of whole-cell lysates from cells infected with GFP or FoxO1-GFP at MOI as indicated. (E) In vitro kinase assay of relative Akt kinase activities in cells overexpressing GFP, FoxO1-GFP, or caFoxO1-GFP. Results are graphed as mean ± SEM from two independent experiments with duplicate samples. * and ** denote P < 0.05 and 0.01, respectively. (F) FoxO does not alter phosphorylation states of p38 MAP kinase, ERK, or JNK.
Protein overexpression studies can be limited by spurious findings that stem from supraphysiological levels of expression. To test this possibility, we evaluated a dose–response relation between FoxO expression and Akt activation. In these experiments, we observed significant increases in Akt phosphorylation even in cells infected with FoxO1 adenovirus at very low titers [multiplicity of infection (MOI) = 1, Fig. 1D]. In vitro kinase assays revealed significant increases in Akt kinase activity in cardiomyocytes overexpressing FoxO1 or caFoxO1 (Fig. 1E). We observed no effects of FoxO overexpression on the phosphorylation levels of p38, p42/44 (ERK) MAP kinases, or Jun-amino-terminal kinase (JNK) (Fig. 1F), suggesting specificity of the pathway.
Next, we tested whether endogenous FoxO was capable of activating Akt. To accomplish this, we treated cells with resveratrol (RVT), an activator of the type III histone deacetylase Sirt1. Deacetylation by Sirt1 stabilizes the FoxO1 protein by preventing its proteasomal degradation (14). In addition, resveratrol increases FoxO1 nuclear translocation and consequent expression of FoxO1 target genes in hepatocytes (31). We observed similar results in cardiomyocytes, where resveratrol increased the nuclear (active) fraction of FoxO1, an effect opposite to that of IGF-1 [supporting information (SI) Fig. 6 A and B]. Resveratrol also increased nuclear FoxO1 in the presence of IGF-1 (SI Fig. 6 A and B), suggesting that FoxO1 deacetylation in cardiac myocytes can override phosphorylation-dependent mechanisms. Further, quantitative real-time PCR showed that resveratrol elicited a significant increase in the mRNA abundance of atrogin-1, a target gene of FoxO1 and FoxO3 (SI Fig. 6C). Sustained treatment with resveratrol (20 μM, 24 h) induced a modest increase in FoxO3 and FoxO1 protein levels as well as in Akt phosphorylation (SI Fig. 6D). In contrast, short-term resveratrol treatment (<12 h) did not have such an effect (SI Fig. 6E). Together, these results suggest that endogenous FoxO, activated by resveratrol, triggers Akt phosphorylation in heart.
Activated Akt Selectively Phosphorylates and Interacts with FoxO Proteins.
To determine the consequences of FoxO-dependent Akt activation, we evaluated multiple Akt targets. As expected, FoxO-induced increases in Akt activity led to an increase in FoxO protein phosphorylation (both endogenous FoxO and overexpressed FoxO1-GFP) without affecting protein abundance (SI Fig. 7A). Furthermore, FoxO-activated Akt had no effect on an Akt-insensitive mutant (caFoxO1-GFP, mutated at Akt consensus sites T24A/S319A), even though the wild-type and mutant FoxO1-GFP were expressed at similar levels (SI Fig. 7A). Surprisingly, phosphorylation of two other Akt target proteins, glycogen synthase kinase-3 (GSK-3) and mammalian target of rapamycin (mTOR), were not increased.
We next tested whether Akt interacts with FoxO using an immobilized antibody that binds preferentially to Akt phosphorylated at serine 473. Akt coprecipitated with FoxO1 proteins in cardiomyocytes expressing FoxO1-GFP but not in control cells expressing GFP (SI Fig. 7B). These results, then, suggest that activated Akt interacts directly with FoxO proteins, leading to their phosphorylation. Consistent with this, Western blot analysis showed that FoxO proteins that coprecipitate with Akt are phosphorylated (SI Fig. 7B). We did not detect interactions between FoxO and Akt in uninfected control cardiomyocytes, where the majority of Akt and FoxO proteins are unphosphorylated (data not shown). Subcellular fractionation experiments in cardiomyocytes expressing either GFP or caFoxO1 showed that Akt was mainly present in cytoplasmic fractions (SI Fig. 7C). As expected, we detected increased levels of phosphorylated Akt in cells expressing caFoxO1. Thus, FoxO-dependent activation of Akt did not trigger its translocation to the nucleus.
FoxO Diminishes Calcineurin Activity Without Affecting Upstream Regulators of Akt.
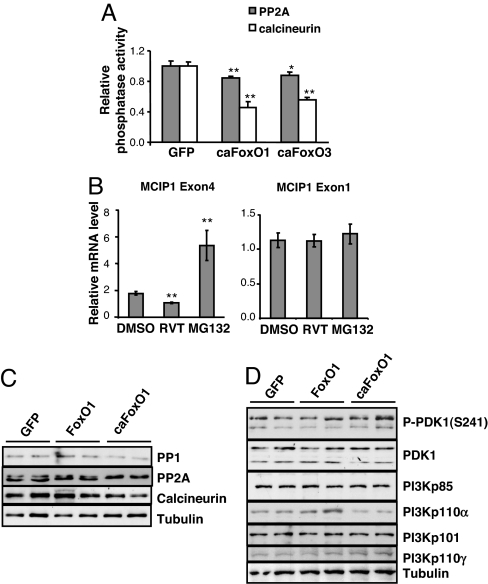
To explore mechanisms underlying FoxO-dependent increases in Akt activity, we evaluated the effects of FoxO on phosphatases and kinases known to target Akt. We first tested the effect of FoxO on PP2A activity (28). Viral expression of constitutively active FoxO1 or FoxO3 led to modest but consistent decreases in PP2A activity (Fig. 2A). In agreement with our previous findings (11), forced expression of active FoxO1 or FoxO3 also significantly inhibited calcineurin phosphatase activity (Fig. 2A). We also tested the effect of resveratrol treatment on PP2A/B activity and found no changes (data not shown). However, using quantitative real-time PCR, a more sensitive assay, we detected in resveratrol-treated cells significant decreases in the mRNA abundance of MCIP1.4, a downstream target of calcineurin, but not that of MCIP1.1, an isoform whose expression is not driven by calcineurin (Fig. 2B). MCIP1.4 mRNA abundance was increased after treatment with MG132, consistent with previous reports that atrogin-1-related proteasomal activity is involved in promoting calcineurin degradation (11, 32).
Fig. 2.
FoxO decreases protein phosphatase activity. Cardiomyocytes were harvested 24 h after adenovirus infection. (A) Relative phosphatase activity was normalized to protein content (experiments repeated three times). Results are graphed as mean ± SEM of a representative experiment with quadruplicate samples. * and ** denote P < 0.05 and 0.01, respectively. (B) Resveratrol treatment decreases mRNA abundance of MCIP1.4 but not MCIP1.1. Data are mean ± SEM (n = 6–8). **, P ≤ 0.01. (C) Western blot of the catalytic subunits of PP1, PP2A, and calcineurin in cells expressing FoxO1-GFP or caFoxO1-GFP. (D) Western blot of the protein abundances of the regulatory (PI3Kp85, PI3Kp101) and catalytic subunits (PI3Kp110α, PI3Kp110γ) of PI3 kinases and the total and phosphorylated protein abundance of PDK1 in cells expressing FoxO1-GFP or caFoxO1-GFP.
Further studies demonstrated that increases in either FoxO1 (Fig. 2C) or FoxO3 (data not shown) activity triggered minimal decreases in the abundance of the catalytic subunits of these protein phosphatases. FoxO overexpression did not alter the protein abundance or phosphorylation of PDK1, nor did it affect the abundance of the major regulatory and catalytic subunits of PI3 kinases in cardiomyocytes (Fig. 2D). These results suggest that FoxO-dependent increases in Akt phosphorylation are caused mainly by suppression of PP2A and calcineurin phosphatase activities.
Akt Is a Target of Calcineurin.
Phospho-Akt is not known to be a substrate of calcineurin phosphatase. Therefore, we examined directly whether perturbation of calcineurin activity affected Akt phosphorylation in cardiomyocytes. Viral overexpression of constitutively active calcineurin (33) triggered a significant decrease in Akt phosphorylation, and overexpression of MCIP1.4, an endogenous inhibitor of calcineurin (34), had the opposite effect (Fig. 3 A and B). Consistent with these results, FK506 (Fig. 3C) and CSA (data not shown), selective inhibitors of calcineurin, caused a significant increase in the level of phosphorylated Akt. Okadaic acid, at a concentration that inhibits both PP2A and PP1 (1 μM) had a stronger effect (Fig. 3C). As expected, inhibition of PP2A alone with low concentrations of okadaic acid (0.2 μM) also increased Akt phosphorylation, and inhibition of all serine/threonine phosphatases with calyculin A had effects greater than inhibiting each phosphatase alone (Fig. 3 E and F). LY294002, a selective inhibitor of PI3 kinase abolished phosphorylation of Akt in the basal state. Also, inhibition of calcineurin by MCIP1.4 or CSA augmented FoxO1-induced increases in Akt phosphorylation when cells were infected at low titer (MOI = 3) (SI Fig. 8A). Inhibitors of calcineurin or PP2A had no effect on the phosphorylation of ERK or p38 under both basal and insulin-stimulated conditions, arguing against nonspecific effects (SI Fig. 8 B and C).
Fig. 3.
Inhibition of calcineurin increases Akt phosphorylation. (A and B) Western blots and densitometric analysis of total or phosphorylated Akt protein levels in extracts from cardiomyocytes infected with adenovirus encoding GFP, constitutively active calcineurin (caCnA), or MCIP1.4. (C–F) Western blots (C and E) and densitometric analyses (D and F) showing effects of phosphatase inhibitors and LY294002. Serum-starved cardiomyocytes were treated for 1 h with okadaic acid (OA, 0.2 or 1 μM), FK506 (10 μM), LY294002 (LY, 10 μM) or for 15 min with calyculin A (CLA, 100 nM). (G) Western blots of whole-cell lysates (WCL, lane 1 and 2) or immunoprecipitated Akt treated with human recombinant calcineurin (lane 3–8) in vitro. Cells were treated with IGF (10 nM, 15min) (lanes 2 and 6–8), and WCL were harvested for Western blotting or for in vitro dephosphorylation assay. Eight (lanes 4 and 7) or 40 (lanes 5 and 8) units of human recombinant calcineurin (BIOMOL) were used. *, P < 0.05 relative to control; **, P < 0.01 relative to control.
To test whether Akt is a direct target of calcineurin, cardiomyocytes were treated with IGF-1, and phosphorylated Akt was immunoprecipitated with an Akt antibody and then exposed to recombinant human calcineurin. Western blot analysis showed that calcineurin reduced levels of phosphorylated Akt in a dose-dependent manner (Fig. 3G). Collectively, these results suggest that Akt is a direct substrate of calcineurin, and reduced calcineurin phosphatase activity contributes, at least in part, to the elevated Akt phosphorylation levels induced by FoxO.
Previous studies suggest that FoxO proteins inhibit calcineurin activity through atrogin-1-mediated proteasomal degradation (10, 11, 32). We reasoned that if proteasomal degradation of calcineurin contributes to FoxO-induced increases in Akt phosphorylation, then inhibition of the ubiquitin-proteasome pathway would prevent the observed increase in Akt phosphorylation. To test this, we exposed cells to the proteasome inhibitor MG132. Relative to control cells, FoxO1 triggered time-dependent increases in Akt phosphorylation (SI Fig. 9 A and B, lanes 1–6). Remarkably, these increases were completely blocked by MG132 (SI Fig. 9 A and B, lanes 7–8). MG132 treatment did not affect basal or IGF-1-induced Akt phosphorylation (SI Fig. 9C), suggesting that upstream activators of Akt are not regulated by proteasomal degradation under these conditions. Consistent with the notion that FoxO-induced increases in Akt phosphorylation are not due to changes upstream of Akt, the PI3 kinase inhibitor LY294002 did not block the enhanced levels of Akt phosphorylation observed in cells expressing caFoxO1 (SI Fig. 9D), whereas it did reduce basal Akt phosphorylation (Fig. 3 C and D).
Calcineurin and PP2A Form a Complex with Akt.
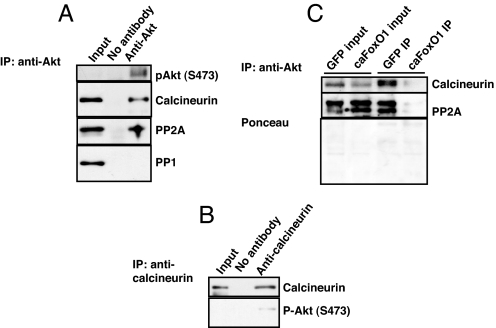
Next, we sought to determine whether endogenous calcineurin interacts directly with Akt. Coimmunoprecipitation analysis showed that anti-Akt antibody precipitated both calcineurin and PP2A (Fig. 4A). However, despite multiple attempts, we were unable to detect interactions between Akt and PP1 (Fig. 4A). In addition, reciprocal coimmunoprecipitation with anti-calcineurin antibody precipitated endogenous phosphorylated Akt (Fig. 4B). We next tested whether FoxO affected the interactions between Akt and calcineurin or PP2A. In cells expressing caFoxO1, we detected lower levels of calcineurin protein associated with FoxO-activated Akt. FoxO overexpression was similarly capable of antagonizing the interaction between Akt and PP2A (Fig. 4C). Similar findings for both calcineurin and PP2A were observed in cells overexpressing caFoxO3 (data not shown). These data, then, provide evidence that calcineurin and PP2A form a complex with Akt. Further, in addition to reducing total phosphatase activity, overexpression of FoxO selectively reduces Akt-associated phosphatase activity.
Fig. 4.
Akt interacts with calcineurin and PP2A in cardiomyocytes. (A and B) Coimmunoprecipitation showing interaction of Akt with calcineurin and PP2A catalytic subunits but not with PP1. Experiments were repeated at least two times with independent cell lysates. (C) Decreased association between calcineurin and Akt or PP2A and Akt in cardiomyocytes overexpressing caFoxO1 relative to that seen in cells overexpressing GFP.
FoxO-Induced Increases in Akt Activity Render Cardiomyocytes Insensitive to Insulin.
We next sought to determine the effect of FoxO-dependent increases in Akt activity on insulin responsiveness. As expected, insulin triggered a robust increase in Akt phosphorylation in cardiomyocytes (Fig. 5A). However, in the presence of overexpressed caFoxO1, insulin-induced increases in Akt phosphorylation were greatly diminished (Fig. 5 A and B). Insulin-dependent phosphorylation of GSK-3 was similarly blunted by FoxO (Fig. 5C). Similar effects were observed with overexpression of wild-type FoxO1 (data not shown) and when different levels of caFoxO1 proteins were expressed in cardiomyocytes (MOI = 1–10, data not shown). Time course analysis of IGF-1-induced responses in cells expressing caFoxO1 proteins revealed both a delay in the onset (15 min in caFoxO1 cells versus 5 min in GFP control cells) and a decrease in the peak response amplitude to insulin (2.4-fold in caFoxO1 cells versus 12-fold in GFP control cells) (Fig. 5 A–C).
Fig. 5.
FoxO attenuates insulin responses in cardiomyocytes. Cardiomyocytes were infected with adenovirus as indicated, and whole-cell lysates were harvested at 24 h after infection. (A and B) Western blots and densitometric analyses showing insulin (Ins, 10 nM) -induced phosphorylation of Akt and GSK-3 in cells expressing GFP or caFoxO1-GFP. Con, vehicle treated control. **, P < 0.01. (C) Time course studies showing IGF-1 (10 nM) -induced phosphorylation of Akt and GSK3 in cells expressing GFP or caFoxO1-GFP. (D) Western blots of insulin-induced Glut4 membrane translocation in cells expressing GFP or caFoxO1-GFP. (E) FoxO inhibits insulin-stimulated glucose uptake. (Upper) Glucose uptake measured 3 and 26 h after cells were infected with GFP or caFoxO1 and subsequently treated with insulin (10 nM, 10min). (Lower) Phosphorylated Akt levels at corresponding time points (experiments repeated twice with triplicate samples). ***, P < 0.001 (*, P < 0.01) vs. GFP without INS; §§, P < 0.01 vs. caFoxO1 without insulin. (F) Western blots showing levels of Akt phosphorylation and protein abundance in adult mouse cardiac myocytes isolated from FoxO3 knockout or wild-type littermates and treated with insulin (INS) (10 nM, 10 min). (G) Proposed working model. IR, insulin receptor.
We next tested whether pharmacological suppression of phosphatases mimics the effects of FoxO on insulin responsiveness. Pretreatment with FK506, okadaic acid, or calyculin A each led to increases in Akt phosphorylation under both basal conditions and on short-term exposure to insulin (SI Fig. 10). Also, overexpression of constitutively active Akt (Myr-Akt) similarly blunted insulin responsiveness (data not shown), consistent with previous reports (35).
In both Drosophila and mammals, FoxO activates transcription of the insulin receptor (IR), an effect that is up-regulated during starvation and leads to insulin sensitization (21, 36). In cardiomyocytes overexpressing FoxO1 and caFoxO1, we found that FoxO had no significant effect on IR protein abundance (SI Fig. 11A), IR transcript levels (SI Fig. 11B), or IRS2 protein levels (SI Fig. 11C).
To investigate whether FoxO-induced increases in Akt activity alter glucose metabolism, we measured insulin-induced glucose uptake. Forced expression of caFoxO1 attenuated insulin-induced membrane translocation of the glucose transporter Glut4 (Fig. 5D), consistent with diminished insulin responsiveness. Further, forced expression of caFoxO1 led to significant decreases in insulin-induced glucose uptake in cardiomyocytes (Fig. 5E). Similar results were observed with caFoxO3 (data not shown).
Finally, to test for effects of endogenous FoxO on insulin signaling, we studied adult cardiac myocytes isolated from FoxO3-null mice [FoxO1-null mice are embryonic lethal (24)]. Basal levels of phospho-Akt were lower in FoxO3-null myocytes relative to wild type (Fig. 5F), consistent with our findings of Akt-activating effects of FoxO in heart. Moreover, exposure to insulin led to substantially greater increases in Akt phosphorylation in FoxO3-null hearts compared with wild type, pointing to insulin sensitization in the FoxO3-null background. Similar results were observed with IGF-1 (data not shown). These data, then, lend additional support to our conclusions that FoxO activity reduces insulin sensitivity in heart.
Discussion
Insulin resistance is a hallmark of type 2 diabetes and several other disorders, including obesity, sepsis, and metabolic syndrome. As a result, insulin resistance contributes significantly to cardiovascular morbidity and mortality (37). Here, we elucidate a mechanism whereby FoxO transcription factors participate in feedback regulation of insulin signaling in cardiac muscle. The major findings we describe are (i) FoxO activates Akt, and resveratrol, which increases endogenous FoxO activity in cardiomyocytes, has similar effects; (ii) FoxO-activated Akt forms a complex with FoxO and signals selectively to some, but not all, downstream targets; (iii) Akt is a binding partner and enzymatic target of calcineurin; (iv) FoxO-dependent declines in calcineurin activity and in Akt–calcineurin interaction together lead to Akt activation; and (v) FoxO activation attenuates insulin signaling and glucose uptake in cardiac myocytes. Together, these results elucidate a previously uncharacterized pathway governing insulin signaling in the myocardium, one where FoxO selectively fine-tunes insulin responsiveness through regulation of Akt activity. A proposed working model is presented (Fig. 5G).
Feedback Regulation of Akt by FoxO.
The transcriptional activity of FoxO proteins is regulated by posttranslational modifications, including phosphorylation, acetylation, and ubiquitylation (13, 38). Here, we report that FoxO1 or FoxO3 also trigger increases in Akt phosphorylation and kinase activity, consistent with prior reports on FoxO3 in heart (10) and FoxO1 in liver (23). However, in contrast with our findings, forced expression of FoxO1 in hepatocytes led to increased phosphorylation of both Akt and GSK-3 (23), two prominent Akt substrates, whereas we find in heart that FoxO-activated Akt does not lead to increases in the phosphorylation state of these proteins. One potential explanation for this discrepancy is that in cardiomyocytes, a sequestered subpopulation of activated Akt is formed—and shielded from certain substrates—as a result of reduced interaction with protein phosphatases and associated scaffolding complexes. Alternatively, differential expression of Akt isoforms in heart and liver may exist, with FoxO preferentially targeting one Akt isoform while leaving others unchecked (39). That FoxO-induced activation of Akt resulted from reduced phosphatase activity rather than increased kinase activity may also account for the apparent discrepancy that PI3 kinase pathway-activated Akt accumulates in the nucleus, and FoxO-activated Akt is localized in the cytosol. Our finding that FoxO is capable of binding and sequestering activated Akt—leaving its activity toward other downstream targets unchecked—uncovers a mechanism for additional fine-tuning of the signaling cascade.
It was reported recently that tribble 3 (Trb3), a pseudokinase and modulator of Akt, participates in FoxO1-induced increases in Akt phosphorylation in the liver (23). However, multiple studies have failed to detect Trb3 in heart (40, 41). Findings reported here point to regulatory mechanisms governing Akt dephosphorylation as central to FoxO-dependent Akt activation. Similar to our findings in cardiac myocytes, Matsumoto et al. (23) did not detect changes in PP2A abundance in FoxO1-transduced hepatocytes. We, however, have extended these findings by testing and inhibiting the enzymatic activities of PP2A (and other) phosphatases, detecting significant FoxO-induced declines. These latter experiments are consistent with our prior report of marked FoxO-dependent decreases in calcineurin/NFAT signaling (11). These declines in phosphatase activity likely stem from proteasomal degradation of calcineurin through the FoxO target protein atrogin-1 (32). Additional evidence for involvement of the proteasome pathway is provided here in experiments where FoxO-dependent increases in Akt phosphorylation were blocked by the proteasome inhibitor MG132.
By inducing Akt phosphorylation, FoxO is capable of activating its own inhibitor. Thus, the existence of simultaneous, opposing, nonequilibrium reactions (a futile cycle) must be considered. However, phosphorylation of FoxO is a powerful, yet only partial, mechanism controlling FoxO transcriptional activity. Furthermore, FoxO activity is governed by other posttranslational modifications; for example, we find that deacetylation is capable of overriding the inhibitory effects of FoxO phosphorylation, consistent with previous reports (42, 43).
Akt Is a Calcineurin Target.
The PI3K/Akt pathway is a key regulator of four intersecting biological processes: cell growth and survival, cell-cycle progression, and metabolism. In heart, Akt contributes to both pathological and physiological cardiac growth, myocyte survival, and contractile function (44). It is not surprising, then, that Akt activity is exquisitely regulated in cardiac tissue. Findings reported here highlight the importance of phosphatase actions on Akt and show that, in addition to PP1, PP2A, and PHLPP (28, 29, 45), calcineurin also dephosphorylates Akt. Interestingly, targeted inhibition of calcineurin or PP2A alone in myocytes provoked only modest increases in Akt phosphorylation; in contrast, combined suppression of PP2A and PP1, or all three serine/threonine phosphatases together, had robust effects, suggesting possible synergistic actions.
We did not detect interaction between Akt and PP1. However, it is possible that PP1 also contributes indirectly to FoxO-induced increases in Akt phosphorylation through its interactions with calcineurin and other phosphatases. For example, calcineurin has been shown to dephosphorylate and inactivate DARPP-32, a potent inhibitor of PP1 (46). Recent reports also showed that both calcineurin and PP2A dephosphorylate and inactivate inhibitor-1, a functional homolog of DARPP-32 in cardiomyocytes (47). Thus, synergy among Akt phosphatases may arise from reduced calcineurin and PP2A activities leading to increased inhibitor-1 function, diminished PP1 activity, and enhanced Akt phosphorylation and function.
FoxO and Insulin Resistance.
In different tissues, FoxO transcriptional activity is capable of either sensitizing or inhibiting insulin responsiveness. For example, in both Drosophila and mammals, FoxO1 triggers insulin sensitization because of its actions to up-regulate expression of the insulin receptor (36). However, there is precedent in multiple other contexts that sustained increases in FoxO activity induce insulin resistance (18, 19). FoxO proteins are known as longevity factors (48, 49), and it has been proposed that insulin resistance at certain levels may extend lifespan by maintaining cellular lipid homeostasis and preventing lipid overload (50). Our evidence in heart provides additional evidence that FoxO-dependent mechanisms regulate insulin responses at multiple points. That dFoxO affects multiple steps in the insulin signaling cascade in Drosophila points to significant evolutionary conservation of this mechanism. Interestingly, in db/db mice, hepatic FoxO is nuclear-localized and Akt is hyperphosphorylated in concert with dysregulated expression of genes controlling gluconeogenesis, a finding that suggests that FoxO-dependent mechanisms contribute to insulin resistance in this model of type 2 diabetes (51).
We detected no changes in insulin responsiveness triggered by resveratrol (data not shown), which we speculate stems from the only modest effects it has on Akt phosphorylation. On the other hand, resveratrol increases the activities of the transcriptional coactivator PGC1α and AMPK (AMP-activated protein kinase), both of which improve insulin sensitivity (52, 53). Also, PGC1α has been shown to suppress FoxO3 action in skeletal muscle (9). Together, these actions may antagonize the FoxO-induced inhibition of insulin responsiveness in cardiomyocytes.
Chronic activation of Akt in heart in vivo results in impaired insulin responsiveness (54), a finding consistent with our results. That said, FoxO may also affect steps in the insulin signaling cascade that are Akt-independent, such as Ca2+/calmodulin-dependent protein kinase kinases (CaMKKs) and AMPK (7, 55). A recent study described a pathway that selectively targets for proteasomal degradation substrates that are phosphorylated (56). Because FoxO reduces phosphatase activities of both PP2A and PP2B, it may increase the phosphorylation state, and subsequent degradation, of proteins that are unrelated to the Akt pathway but that are also involved in insulin responsiveness.
Perspective.
Cardiometabolic risk encompasses a cluster of risk factors that predispose individuals to type 2 diabetes and premature cardiovascular disease. In the great majority of instances, insulin resistance is a major underlying mechanism. Here, we describe a mechanism whereby FoxO transcription factors participate in feedback regulation of insulin signaling in cardiac muscle, providing an additional echelon of control to calibrate this critical pathway. As a result, these findings prompt consideration of FoxO as a target of therapeutic intervention in metabolic syndrome.
Materials and Methods
Primary Cardiac Cell Preparation.
Neonatal rat ventricular myocytes were isolated from the ventricles of postnatal day 1–2 Sprague–Dawley rats (11).
Statistics.
Unless otherwise specified, data are expressed as mean ± SEM, triplicate samples were used, and each experiment was repeated at least twice. Statistical significance was analyzed with a Student's unpaired t test or one-way ANOVA, followed by Bonferroni's method for post hoc pairwise multiple comparisons. Additional materials and methods are provided in SI Materials and Methods.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Drs. Stephen J. Gold and James Bibb for helpful discussions, Drs. Diego Castrillon (University of Texas Southwestern) and Ronald DePinho (Harvard University, Cambridge, MA) for providing FoxO3-null mice, and Preston Wong and Jun Cheng for technical assistance. This work was supported by grants from the Donald W. Reynolds Foundation (to J.A.H.); National Institutes of Health Grants HL-075173, HL-006296, and HL-080144 (to J.A.H.) and HL-072016 (to B.A.R.); and American Heart Association Grants 0640084N (to J.A.H.), 0565100Y (to Y.G.N.), and 0655202Y (to B.A.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610290104/DC1.
References
- 1.Eckel RH, Grundy SM, Zimmet PZ. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.Paternostro G, Camici PG, Lammerstma AA, Marinho N, Baliga RR, Kooner JS, Radda GK, Ferrannini E. J Clin Invest. 1996;98:2094–2099. doi: 10.1172/JCI119015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poornima IG, Parikh P, Shannon RP. Circ Res. 2006;98:596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- 4.Boudina S, Abel ED. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 5.Paternostro G, Pagano D, Gnecchi-Ruscone T, Bonser RS, Camici PG. Cardiovasc Res. 1999;42:246–253. doi: 10.1016/s0008-6363(98)00233-8. [DOI] [PubMed] [Google Scholar]
- 6.Doenst T, Goodwin GW, Cedars AM, Wang M, Stepkowski S, Taegtmeyer H. Metab Clin Exp. 2001;50:1083–1090. doi: 10.1053/meta.2001.25605. [DOI] [PubMed] [Google Scholar]
- 7.Arad M, Seidman CE, Seidman JG. Circ Res. 2007;100:474–488. doi: 10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- 8.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 9.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skurk C, Izumiya Y, Maatz H, Razeghi P, Shiojima I, Sandri M, Sato K, Zeng L, Schiekofer S, Pimentel D, et al. J Biol Chem. 2005;280:20814–20823. doi: 10.1074/jbc.M500528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, Cheng J, Lu G, Morris DJ, Castrillon DH, et al. Circulation. 2006;114:1159–1168. doi: 10.1161/CIRCULATIONAHA.106.637124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran H, Brunet A, Griffith EC, Greenberg ME. SCI STKE Mar. 2003;4:RE5. doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- 13.Accili D, Arden KC. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 14.van der Horst A, Burgering BM. Nat Rev Mol Cell Biol. 2007;8:440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 15.Huang H, Tindall DJ. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 16.Barthel A, Schmoll D, Unterman TG. Trends Endocrinol Metab. 2005;16:183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, et al. J Biol Chem. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
- 19.Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, Mochida K, Hata T, Matsuda J, Aburatani H, et al. J Biol Chem. 2004;279:41114–41123. doi: 10.1074/jbc.M400674200. [DOI] [PubMed] [Google Scholar]
- 20.Watamoto K, Towatari M, Ozawa Y, Miyata Y, Okamoto M, Abe A, Naoe T, Saito H. Oncogene. 2003;22:9176–9184. doi: 10.1038/sj.onc.1206902. [DOI] [PubMed] [Google Scholar]
- 21.Puig O, Tjian R. Genes Dev. 2005;19:2435–2446. doi: 10.1101/gad.1340505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakae J, Biggs WH, III, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D. Nat Genet. 2002;32:245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto M, Han S, Kitamura T, Accili D. J Clin Invest. 2006;116:2464–2472. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 25.Matsuzaki H, Daitoku H, Hatta M, Tanaka K, Fukamizu A. Proc Natl Acad Sci USA. 2003;100:11285–11290. doi: 10.1073/pnas.1934283100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stokoe D, Stephens LR, Copeland T, Gaffney PRJ, Reese CB, Painter GF, Holmes AB, McCormick F, Hawkins PT. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 27.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 28.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Gao T, Furnari F, Newton AC. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Zdychova J, Komers R. Physiol Res. 2005;54:1–16. doi: 10.33549/physiolres.930582. [DOI] [PubMed] [Google Scholar]
- 31.Frescas D, Valenti L, Accili D. J Biol Chem. 2005;280:20589–20595. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
- 32.Li HH, Kedar V, Zhang C, McDonough H, Arya R, Wang DZ, Patterson C. J Clin Invest. 2004;114:1058–1071. doi: 10.1172/JCI22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh M, Rybkin II, Copeland V, Czubryt MP, Shelton JM, van Rooij E, Richardson JA, Hill JA, De Windt LJ, Bassel-Duby R, et al. Mol Cell Biol. 2005;25:6629–6638. doi: 10.1128/MCB.25.15.6629-6638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vega RB, Yang J, Rothermel BA, Bassel-Duby R, Williams RS. J Biol Chem. 2002;277:30401–30407. doi: 10.1074/jbc.M200123200. [DOI] [PubMed] [Google Scholar]
- 35.Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, Ogawa W, del Monte F, Gwathmey JK, Grazette L, et al. J Clin Invest. 2005;115:2128–2138. doi: 10.1172/JCI23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puig O, Tjian R. Cell Cycle. 2006;5:503–505. doi: 10.4161/cc.5.5.2501. [DOI] [PubMed] [Google Scholar]
- 37.Accili D. Diabetes. 2004;53:1633–1642. doi: 10.2337/diabetes.53.7.1633. [DOI] [PubMed] [Google Scholar]
- 38.Vogt PK, Jiang H, Aoki M. Cell Cycle. 2005;4:908–913. doi: 10.4161/cc.4.7.1796. [DOI] [PubMed] [Google Scholar]
- 39.Muslin AJ, DeBosch B. Novartis Found Symp. 2006;274:118–126. discussion 126–131, 152–155, 272–276. [PubMed] [Google Scholar]
- 40.Kiss-Toth E, Bagstaff SM, Sung HY, Jozsa V, Dempsey C, Caunt JC, Oxley KM, Wyllie DH, Polgar T, Harte M, et al. J Biol Chem. 2004;279:42703–42708. doi: 10.1074/jbc.M407732200. [DOI] [PubMed] [Google Scholar]
- 41.Ord D, Ord T. Exp Cell Res. 2003;286:308–320. doi: 10.1016/s0014-4827(03)00070-3. [DOI] [PubMed] [Google Scholar]
- 42.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 43.Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Shiojima I, Walsh K. Genes Dev. 2006;20:3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 45.Jiang X, Kim HE, Shu H, Zhao Y, Zhang H, Kofron J, Donnelly J, Burns D, Ng SC, Rosenberg S, et al. 2003;299:223–226. doi: 10.1126/science.1076807. [DOI] [PubMed] [Google Scholar]
- 46.Hemmings HC, Jr, Nairn AC, Greengard P. J Biol Chem. 1984;259:14491–14497. [PubMed] [Google Scholar]
- 47.El-Armouche A, Bednorz A, Pamminger T, Ditz D, Didie M, Dobrev D, Eschenhagen T. Biochem Biophys Res Commun. 2006;346:700–706. doi: 10.1016/j.bbrc.2006.05.182. [DOI] [PubMed] [Google Scholar]
- 48.Greer EL, Brunet A. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 49.Morris BJ. J Hypertens. 2005;23:1285–1309. doi: 10.1097/01.hjh.0000173509.45363.dd. [DOI] [PubMed] [Google Scholar]
- 50.Unger RH. Nat Med. 2006;12:56–57. doi: 10.1038/nm0106-56. [DOI] [PubMed] [Google Scholar]
- 51.Aoyama H, Daitoku H, Fukamizu A. Int J Mol Med. 2006;18:433–439. [PubMed] [Google Scholar]
- 52.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 54.Matsui T, Nagoshi T, Hong EG, Luptak I, Hartil K, Li L, Gorovits N, Charron MJ, Kim JK, Tian R, et al. Am J Physiol. 2006;290:E789–E797. doi: 10.1152/ajpendo.00564.2004. [DOI] [PubMed] [Google Scholar]
- 55.Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. Diabetes. 2007;56:1403–1409. doi: 10.2337/db06-1230. [DOI] [PubMed] [Google Scholar]
- 56.Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. Science. 2006;314:467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.