Abstract
Endocannabinoids (eCBs) mediate short- and long-term depression of synaptic strength by retrograde transsynaptic signaling. Previous studies have suggested that an eCB mobilization or release step in the postsynaptic neuron is involved in this retrograde signaling. However, it is not known whether this release process occurs automatically upon eCB synthesis or whether it is regulated by other synaptic factors. To address this issue, we loaded postsynaptic striatal medium spiny neurons (MSNs) with the eCBs anandamide (AEA) or 2-arachidonoylglycerol and determined the conditions necessary for presynaptic inhibition. We found that presynaptic depression of glutamatergic excitatory postsynaptic currents (EPSCs) and GABAergic inhibitory postsynaptic currents (IPSCs) induced by postsynaptic eCB loading required a certain level of afferent activation that varied between the different synaptic types. Synaptic depression at excitatory synapses was temperature-dependent and blocked by the eCB membrane transport blockers, VDM11 and UCM707, but did not require activation of metabotropic glutamate receptors, l-calcium channels, nitric oxide, voltage-activated Na+ channels, or intracellular calcium. Application of the CB1R antagonist, AM251, after depression was established, reversed the decrease in EPSC, but not in IPSC, amplitude. Direct activation of the CB1 receptor by WIN 55,212-2 initiated synaptic depression that was independent of afferent stimulation. These findings indicate that retrograde eCB signaling requires a postsynaptic release step involving a transporter or carrier that is activated by afferent stimulation/synaptic activation.
Keywords: anandamide, basal ganglia, synaptic plasticity, CB1 receptor
Changes in synaptic strength, such as depolarization-induced suppression of excitatory (DSE) or depolarization-induced suppression of inhibitory (DSI) transmission and long-term depression (LTD), can be induced by retrograde endocannabinoid (eCB) signaling (1–3). eCB production and release can be triggered by depolarization (4–6) or neurotransmitter-induced increases in intracellular calcium concentration and after activation of G protein-coupled receptors (7, 8) or voltage-gated calcium channels (9). Postsynaptically released eCBs produce synaptic depression presumably by binding to presynaptic cannabinoid 1 receptors (CB1Rs) and decreasing the probability of neurotransmitter release either in a transient or sustained manner (2, 3, 10–12).
The two best characterized eCBs are N-arachidonoylethanolamide [anandamide (AEA)] and 2-arachidonoylglycerol (2-AG), which are synthesized from membrane-derived lipid precursors (13, 14). Diffusion and cellular uptake of both AEA and 2-AG are believed to be facilitated by an AEA transporter (AMT) (6, 8, 15–18), although it has been suggested that there is no need for such a transporter (19, 20). AEA transport is cell-specific, unaffected by metabolic inhibitors, temperature-dependent, energy- and sodium-independent, and believed to involve a protein carrier molecule (18, 21). Inhibitors of the AMT, such as AM404 and VDM11, act as competitive substrates for the putative transporter (22, 23). AM404 prevents intracellular accumulation of extracellularly applied AEA (21), and intracellular loading of AM404 or VDM11 prevents increased pair-pulse ratio (PPR) induced by postsynaptic AEA or 2-AG loading, as well as synaptic depression induced by high-frequency stimulation in the striatum (2) and timing-dependent LTD in the somatosensory cortex (15). Two other selective eCB transport inhibitors, OMDM-2 and UCM707, also block DSE in the ventral tegmental area (VTA) (6) and increase stimulus-evoked firing of dopaminergic neurons in the VTA (24). Biochemical evidence of the inhibitory effect of blockers on eCB release also has been published (25, 26).
Repetitive activation of afferent fibers and synapses can induce eCB retrograde signaling through stimulation of mechanisms needed for postsynaptic eCB synthesis (3, 12, 27–31) and by providing a synergistic presynaptic signal necessary for LTD induction (2, 28, 32–34). However, it is not clear whether afferent/synaptic activation plays any role in the eCB release process. Considering the important role for eCBs in retrograde synaptic signaling, it is vital to understand how eCBs are released from the postsynaptic cell. By loading striatal MSNs with AEA or 2-AG, we can bypass eCB synthesis and more directly assess the release process. Using this approach, we show that eCB signaling is stimulated by afferent activation and the threshold for stimulus-induced release varies between excitatory and inhibitory synapses. We also show that synaptic depression induced by explicit activation of CB1R with an extracellularly applied agonist does not require afferent/synaptic activation. Our data suggest that eCB signaling requires a postsynaptic release step that is regulated by synaptic activation.
Results
eCB-Induced Depression of EPSC Amplitude Depends on Afferent Activation.
Consistent with our previous findings (2, 30), we observed that postsynaptic loading of the eCBs AEA or 2-AG produced depression of synaptic transmission at glutamatergic synapses onto MSNs (2, 30) during recordings from neurons clamped at −50 mV. In all neurons, regardless of the presence or absence of postsynaptic eCB loading, we observed an increase in EPSC amplitude just after initiating the whole-cell recording. EPSC response amplitude stabilized within 5 min after initiating the recording (Fig. 1a). After this stabilization period, a decrease in EPSC amplitude was detected within 10 min of the initiation of paired-pulse stimulation in >70% (17 of 23) of AEA-loaded cells (EPSC amplitude at t = 15 min in affected cells = 62 ± 7.5% of baseline; n = 10; P < 0.001) (Fig. 1a), whereas no depression in EPSC amplitude was detected during single-pulse stimulation in any cell (EPSC amplitude at t = 15 min = 104 ± 7.5%; n = 10; P > 0.05; paired- vs. single-pulse protocol P < 0.001) (Fig. 1a). The level of stimulation required appeared to be predominantly dependent on the interpulse interval because AEA loading, combined with stimulation at 0.1 Hz, which evokes the same number of EPSCs as the 50-mHz paired-pulse protocol, was insufficient to induce a depression in EPSC amplitude (amplitude at t = 20–25 min = 100 ± 14%; n = 6; P > 0.05). Synaptic depression was not observed in any MSNs in slices stimulated with a paired-pulse protocol without intracellular postsynaptic loading of AEA (Fig. 1a). The change in EPSC amplitude produced by postsynaptic loading/afferent activation was calculated from this baseline, and the relative response amplitude 20–25 min after achieving a stable baseline is presented unless otherwise stated.
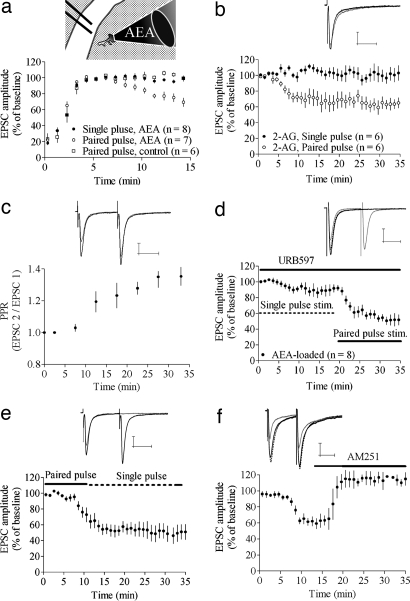
Fig. 1.
eCB release depends on afferent activation. (a) Intracellular loading with AEA induced a depression in EPSC amplitude during paired-pulse, but not single-pulse, stimulation. The time for initial stabilization of EPSC amplitude after establishing the whole-cell recording was similar in AEA-loaded and control cells. Thus, the time courses for EPSC data in subsequent figures are shown starting from the time at which the response amplitude stabilized. (Inset) Schematic drawing of AEA loading into an MSN in the dorsolateral part of the striatum. Afferent activation was given through a bipolar electrode placed in the overlying white matter. (b) Postsynaptic loading of 2-AG induced a depression similar to that induced by AEA. (c) The depression in EPSC amplitude induced by AEA was concomitant with an increase in PPR. Normalized PPR values from nine cells are shown. (d) Treatment with 1 μM URB597, the FAAH blocker, slightly enhanced depression induced by AEA during single-pulse stimulation, but paired-pulse stimuli was required to induce a robust depression. (e) Paired-pulse stimulation was required for induction, but not maintenance, of AEA-induced depression (n = 6). (f) AEA-induced depression was completely reversed by 3 μM AM251, the CB1R antagonist (n = 5). Example trace shows baseline EPSC (black line) after t = 10–15 min (gray line) and after 10–15 min of AM251 treatment (dashed line). All other example traces show baseline EPSC (black lines) and after 20–25 min of single-pulse stimulation (gray lines). EPSC amplitude data are mean ± SEM. (Calibration bars, 25 msec and 100 pA.)
2-AG-induced depression exhibited a time course similar to that of AEA-induced depression [EPSC amplitude = 69 ± 12%; n = 6; P < 0.05 (paired pulse); 103 ± 14%; n = 6 (single pulse); paired pulse vs. single pulse protocol P < 0.05] (Fig. 1b). In subsequent experiments, only neurons loaded with AEA were examined.
The change in EPSC amplitude occurred in parallel with a gradual increase in PPR (Fig. 1c), which supports presynaptic expression and a role for decreased release probability in AEA-induced depression (normalized PPR 25 min after stabilization of response amplitude = 1.21 ± 0.07; n = 13; P < 0.001). It is possible that a significant proportion of the postsynaptically loaded AEA and 2-AG are hydrolyzed by the fatty acid amide hydrolase (FAAH). We addressed this issue by extracellular application of 1 μM URB597, an FAAH inhibitor. In the presence of URB597, AEA loading induced a small, but significant, depression during single-pulse stimulation (EPSC amplitude at t = 15–20 min = 91 ± 6.3%; n = 8; P < 0.05), but robust depression was only seen after paired-pulse stimulation (EPSC amplitude after 10–15 min paired-pulse stimulation = 65 ± 8.5%; P < 0.001). FAAH inhibition did not enhance AEA-induced depression during paired-pulse stimulation (P > 0.05) (Fig. 1d).
To evaluate whether paired-pulse stimulation was required for maintained depression or initiation of the depression, AEA-loaded MSNs were activated with paired-pulse stimulation for 15 min, followed by single-pulse stimulation for an additional 25 min. The AEA-induced depression in EPSC amplitude was not reversed during single-pulse stimulation within this time frame, suggesting that strong afferent activation is not crucial for the maintenance of AEA depression (EPSC amplitude = 54 ± 15%; n = 6; P > 0.001) (Fig. 1e). AEA-induced depression was reversed by application of AM251 after the decrease in EPSC amplitude was established (EPSC amplitude after 25–30 min = 112 ± 16%; n = 6; P > 0.05) (Fig. 1f), showing that eCB-induced depression is similar to reversible depression produced by CB1R agonist application (2, 35), and AEA loading does not induce irreversible LTD.
AEA-Induced Depression Exhibits Properties Consistent with a Transport-Like Process.
Depression induced by postsynaptic AEA loading plus paired-pulse stimulation was significantly reduced at room temperature (RT) (20–22°C), relative to the depression observed at a higher temperature (AEA 30°C vs. RT) (P < 0.001) (Fig. 2a). At RT, EPSC amplitude was not reduced at t = 20–25 min (90 ± 11%; n = 8; P > 0.05), but a slight depression was seen after 30–35 min of paired-pulse afferent stimulation (85 ± 14%; n = 8; P < 0.05). Postsynaptic loading with VDM11 or UCM707, blockers of the putative eCB transporter, prevented the synaptic depression induced by combined postsynaptic AEA application and paired-pulse stimulation [EPSC amplitude = 103 ± 16.5%; n = 8; P > 0.05 (VDM11);105 ± 9.6%; n = 7; P > 0.05 (UCM707)] (Fig. 2a) (2).
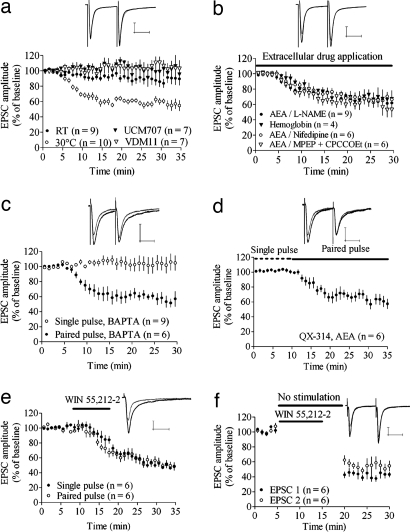
Fig. 2.
Molecular mechanisms of AEA loading-induced depression and differences relative to agonist-induced depression. (a) AEA-induced depression was blocked by postsynaptic loading of 10 μM VDM11 and 10 μM UCM707, the AMT blockers, and decreased at RT. Example traces show EPSCs at baseline and after 20–25 min of paired-pulse stimulation in MSNs loaded with AEA and UCM707. (b) Perfusion of 100 μM l-NAME, the NO synthetase inhibitor, or 500 mg/liter hemoglobin, the NO scavenger, did not prevent AEA-induced depression. EPSC amplitude also was depressed in AEA-loaded MSNs during treatment with 40 μM MPEP and 80 μM CPCCOEt, the group 1 mGluR antagonists, and during blockade of l-type calcium channels with 20 μM nifedipine. Example traces show EPSCs at baseline and after 20–25 min of paired-pulse stimulation during perfusion of l-NAME. (c) Chelation of intracellular calcium with 20 mM BAPTA did not prevent AEA-induced depression during paired-pulse stimulation and did not facilitate depression during single-pulse stimulation. (d) Blockade of voltage-gated sodium channels by 6 μM QX-314 did not affect AEA-induced depression. (e and f) Synaptic depression induced by activation of the CB1 receptor with extracellular WIN 55,212-2 is independent of afferent activation. Example traces show EPSC amplitude at the time point where paired-pulse stimulation started (t = 10 min) (black lines) and after 20–25 min of paired-pulse stimulation (gray lines). EPSC amplitude data are mean ± SEM. (Calibration bars, 25 msec and 100 pA.)
AEA-Induced Depression During Paired-Pulse Stimulation.
We next evaluated whether mechanisms implicated in eCB retrograde signaling might underlie the requirement for strong afferent activation in depression. AEA-induced depression of EPSC amplitude did not appear to involve the activation of postsynaptic metabotropic group I receptors (mGluRs) because a robust depression could be induced in slices perfused with 40 μM MPEP plus 80 μM CPCCOEt (EPSC amplitude = 66 ± 12%; n = 7; P < 0.001) (Fig. 2b). Activation of l-type calcium channels has been shown to be pivotal for LTD induction (9, 36, 37), but perfusion with 20 μM nifedipine did not affect AEA-induced depression in EPSC amplitude (65 ± 7.2%; n = 6; P < 0.001) (Fig. 2b), showing that l-type calcium channels are not involved at a stage when eCB levels are already high. Nitric oxide (NO) has been reported to enhance the activity of the AMT (16, 38–40). However, neither inhibition of NO production by 100 μM Nω-Nitro-l-arginine methyl ester hydrochloride (l-NAME) nor 500 mg/liter hemoglobin, the NO scavenger, was able to block AEA-induced depression [EPSC amplitude = 66 ± 7.9%; n = 7; P < 0.001 (l-NAME); 66 ± 11%; n = 4; P < 0.05 (hemoglobin)] (Fig. 2b). eCB mobilization in the hippocampus has been suggested to be dependent on activation of ryanodine receptors (41), and it is possible that eCB release requires elevated calcium levels in the postsynaptic cell. However, the depression in EPSC amplitude also remained intact during postsynaptic loading with 20 mM AEA and BAPTA (61 ± 8.7%; n = 6; P < 0.001) (Fig. 2c). Chelation of intracellular calcium did not alter AEA-induced depression during single-pulse stimulation (103 ± 18%; n = 8; P > 0.05) (Fig. 2c), indicating that a low level of intracellular calcium also is not a signal that enhances eCB release. The depression in EPSC amplitude caused by postsynaptic AEA was unaffected by intracellular loading of 6 μM QX-314, a blocker of voltage-dependent sodium channels. In these experiments, EPSCs were induced by single-pulse stimulation for the first 15 min to extend the time for QX-314 to block sodium channels (EPSC amplitude after 20–25 min of paired-pulse stimulation = 61 ± 13%; n = 7; P < 0.001) (Fig. 2d).
eCB Release and Membrane Potential.
We next evaluated possible effects of membrane potential on AEA-induced depression in EPSC amplitude. MSNs clamped at −50 or −70 mV were loaded with AEA and stimulated with a paired- or single-pulse stimulation protocol. The depression in EPSC amplitude after paired-pulse stimulation was independent of holding potential [EPSC amplitude = 63 ± 3.1% of control; n = 9; P < 0.001 (−50 mV); 64 ± 7.5% of control; n = 6; P < 0.001 (−70 mV); Hp −50 mV vs. Hp −70 mV P > 0.05] [supporting information (SI) Fig. 4]. Single-pulse stimulation did not induce a depression in EPSC amplitude in AEA-loaded MSNs clamped at −50 mV (EPSC amplitude = 98 ± 8.6% of control; n = 6; P > 0.05) or −70 mV (EPSC amplitude = 90 ± 9.6% of control; n = 11; P > 0.05) (SI Fig. 4). However, a small rundown was detected in AEA-loaded MSNs examined when using a CsCl-based internal solution clamped at −50 mV and stimulated with a single-pulse protocol (EPSC amplitude = 89 ± 7.6% of control; n = 13; P < 0.01) (SI Fig. 4). EPSC amplitude evoked by single-pulse stimulation was not depressed after changing the holding potential from −50 to −30 mV for 2 min, which shows that strong depolarization cannot substitute for synaptic activation in inducing eCB signaling (EPSC amplitude = 96 ± 8.6% of control; n = 9; P > 0.05).
Synaptic Depression Induced by Extracellular CB1 Agonist Is Independent of Afferent Activation.
To determine whether afferent activation was required for eCB release, activation of CB1R, or mechanisms downstream of CB1R activation, we treated the slices with 1 μM WIN 55,212-2, a CB1R agonist. Application of WIN 55,212-2 induced a depression in EPSC amplitude that was independent of the pattern of afferent activation [EPSC amplitude = 63 ± 12%; n = 6; P < 0.01 (paired pulse); 64 ± 8.0%; n = 6; P < 0.001 (single pulse); single vs. paired P > 0.05] (Fig. 2e). Furthermore, WIN 55,212-2 also induced a depression of EPSC amplitude when stimulation was suspended for 15 min, and the magnitude of this depression was not further increased when afferent activation was resumed (EPSC amplitude at t = 20 min = 43% ± 8.9% of baseline; n = 6; P < 0.001; EPSC amplitude at t = 30 min = 40 ± 8.2%; P < 0.001; t = 20 min vs. t = 30 min; P > 0.05) (Fig. 2f), showing that activation of the CB1 receptor, or downstream mechanisms involved in presynaptic inhibition, is independent of afferent stimulation.
AEA-Induced Depression at Inhibitory Synapses.
To determine whether postsynaptic AEA loading- and stimulation-induced synaptic depression is restricted to glutamatergic synapses, we next examined GABAergic inhibitory transmission. Afferent stimulation in the presence of ionotropic glutamate receptor antagonists elicited IPSCs that were completely blocked by 50 μM picrotoxin (SI Fig. 5). Postsynaptic AEA loading depressed GABA-mediated IPSCs in a high proportion of cells (24 of 28), indicating that this form of depression does not require activation of ionotropic glutamate receptors. Similar to stabilization of EPSC amplitude, the run-up time for IPSCs was <5 min, and AEA-induced depression was concomitant with a change in PPR (normalized PPR at t = 20–25 min = 1.27 ± 0.19; n = 12; P < 0.05). In contrast to excitatory synapses, AEA-induced depression in IPSC amplitude was independent of the pattern of afferent activation. No significant difference was observed in the depression with the two stimulus paradigms (P > 0.05) [IPSC amplitude = 53 ± 5.2%; n = 6; P < 0.001 (paired pulse); 51 ± 8.1%; n = 8; P < 0.001 (single pulse)] (Fig. 3a).
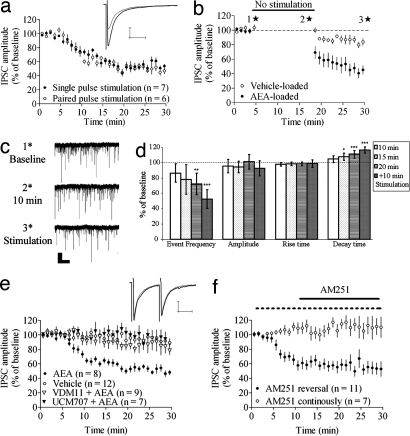
Fig. 3.
AEA-induced depression at GABAergic synapses. (a) AEA-induced depression at inhibitory synapses is independent of stimulation protocol. (b) IPSC amplitude also is depressed compared with baseline and vehicle when afferent activation is suspended but is further reduced after stimulation is resumed. (c) Representative traces showing sIPSCs at time points marked with stars in b. (Scale bar: 5 sec and 30 pA.) (d) Statistics for sIPSCs measured continuously in AEA-loaded MSNs, with afferent activation initiated 20 min after establishing the whole-cell recording configuration. Event frequency was significantly reduced after 20 min of postsynaptic AEA loading without afferent activation but decreased further subsequent to paired-pulse activation. (e) AEA-induced depression was prevented by postsynaptic loading of the AMT blockers VDM11 or UCM707. Example traces show IPSC at baseline and after 20–25 min of paired-pulse stimulation in MSNs loaded with AEA and UCM707. (f) Postsynaptic loading combined with afferent activation did not depress IPSCs during continuous AM251 perfusion but previously established depression was not reversed by a 20-min application of AM251. EPSC amplitude data are mean ± SEM. (Calibration bars, 25 msec and 100 pA.)
To determine whether IPSCs could be depressed without afferent activation, stimulation was suspended as soon as a stable baseline response amplitude was achieved and was resumed after a 15-min no-stimulation period. IPSC amplitude was significantly depressed, compared with baseline and vehicle-loaded cells, just after stimulation with paired pulses was resumed (IPSC amplitude at t = 19 min = 65 ± 17%; n = 10; P < 0.01), but continued to decrease with repeated stimulation (IPSC amplitude at t = 30 min = 47 ± 13%; P < 0.001; t = 19 min vs. t = 30 min; P < 0.01) (Fig. 3b). IPSC amplitude was not significantly affected in vehicle-loaded MSNs (IPSC amplitude = 93 ± 8.4%; n = 14; P > 0.05) (Fig. 3e) or vehicle-loaded MSNs treated with AM251 (IPSC amplitude after 15 min of AM251 = 90 ± 9.4%; n = 7; P > 0.05). However, a small rundown in IPSC amplitude was detected when stimulation was suspended for 15 min (IPSC amplitude 20–25 min after established baseline = 88 ± 3.4%; n = 7; P > 0.01) (Fig. 3b).
In the same group of cells used in the experiment described earlier, spontaneous IPSCs (sIPSCs) were measured after the stable baseline was recorded after the 15-min no-stimulus period and after 10 min of additional paired-pulse stimulation (time points are marked with stars in Fig. 3b). The sIPSC frequency was reduced after the 15-min diffusion period (65 ± 15% of control; P < 0.01) and did not decrease further with additional stimulation (66 ± 16% of control; P < 0.05).
Because achieving the initial baseline requires afferent activation, it is possible that eCB release is initiated during baseline recording. Therefore, we measured sIPSCs in AEA-loaded MSNs without examining evoked IPSCs, thus obviating the need for stimulation in the initial part of the recording (n = 11) (Fig. 3d). The sIPSCs were first measured for a 1.5-min period, beginning 5 min after establishing the whole-cell patch-clamp configuration, and every 5 min for a total of 20 min before proceeding with 10 min of paired-pulse stimulation. Event frequency was significantly reduced after 20 min of recording without stimulation, suggesting that eCB release and signaling can occur independent of afferent activation at inhibitory synapses. However, 10 min of paired-pulse stimulation further reduced the frequency of events (P < 0.001), indicating that, although eCBs are released without stimulation, the rate or amount of release is significantly enhanced by afferent activation. Amplitude and rise time of sIPSCs were not affected by AEA loading, but decay time was significantly longer starting from t = 15 min after recording onset (Fig. 3d). sIPSC frequency, amplitude, and rise time were not affected after 15 or 30 min in vehicle-loaded MSNs, but decay time was significantly longer 30 min after establishing the whole-cell recording (111 ± 8.3% of control; n = 6; P < 0.05), indicating that this effect is a consequence of prolonged whole-cell recording, rather than a specific effect of AEA loading.
Similar to glutamatergic synapses, AEA-induced depression was independent of mGluR activation or NO synthesis [IPSC amplitude = 58 ± 17% of control; n = 6; P < 0.01 (80 μM CPCCOEt + 40 μM MPEP); 50 ± 14% of control; n = 5; P < 0.01 (100 μM l-NAME])] (SI Fig. 6), but attenuated by inhibitors of the AMT [IPSC amplitude = 82 ± 13% of control; n = 9; P < 0.05 (10 μM VDM11); 97 ± 14% of control; n = 7; P > 0.05 (10 μM UCM707)] (Fig. 3e). In contrast to glutamatergic synapses, the depression established by AEA loading was not reversed by 3 μM AM251 within the duration of antagonist exposure that we used (IPSC amplitude = 63 ± 13% of control; n = 10; P < 0.01). However, application of AM251 throughout the recording prevented AEA loading-induced IPSC depression (IPSC amplitude = 115 ± 16% of control; n = 8; P > 0.05) (Fig. 3f).
Discussion
By loading MSNs with eCBs, we have shown that eCB release or mobilization depends on the level of afferent/synaptic activation at excitatory synapses in the striatum. Reduced EPSC amplitude occurred during paired-pulse, but not single-pulse, stimulation, indicating that AEA mobilization or release increases with increasing synaptic activation, and these mechanisms occur after the synthesis of the eCB messenger.
Our data are most consistent with the idea that eCB release at excitatory synapses during paired-pulse stimulation involves a transport or carrier-like process, consistent with previous evidence for AMT involvement in eCB release (42). AEA-induced depression of EPSC amplitude was decreased when the temperature was reduced, and depression was inhibited by intracellular loading with the AMT blockers, VDM11 and UCM707, which is in line with previous reports on AEA release and uptake (2, 18, 21, 25, 26). The finding by Van der Stelt et al. (43), that AMT blockers produce cellular buildup of AEA, provides additional support for transporter-mediated release. Furthermore, the fact that AEA-induced depression required paired-pulse stimulation does not support the theory that eCB transport is solely dependent on the concentration gradient across the membrane. Rather, our data suggest that eCB release is not only dependent on biosynthesis and the transmembrane eCB gradient, but also involves a specific, possibly protein-mediated, process (25). Strong afferent stimulation was not required to maintain AEA-induced depression once initiated. It is possible that afferent/synaptic activation has a priming action such that the AMT needs to be activated for a short time period, which then facilitates maintained eCB release. It also is possible that the initiation of depression requires a higher concentration of eCBs, compared with maintaining an established depression. Importantly, AEA-induced depression during paired-pulse stimulation was reversed by the CB1R antagonist, AM251. This finding shows that postsynaptic AEA loading induces reversible synaptic depression that is distinguishable from LTD (2, 35). This depression appears to involve sustained CB1R activation and likely persists only as long as extracellular eCB is present.
Synaptically driven eCB signaling has been suggested to require activation of group I mGluRs and phospholipase C β (44), and thus mGluRs are promising targets for the synaptic activation that participates in eCB release. However, AEA-induced depression was unaltered during group I mGluR blockade. The role of mGluRs and PLC is probably to stimulate eCB production, and these molecules are not required at a stage where eCBs already have been produced (13). Because AEA also induced depression at GABAergic synapses when ionotropic glutamate receptors were blocked, activation of the AMT appears to occur independently of glutamate receptor activation. GABAA receptors also are not obligatory because we observed depression of EPSCs when picrotoxin was included in the aCSF (2). NO has been reported to regulate AMT activity (40), but treatments that block NOS or scavenge NO did not alter AEA-induced depression, indicating that this messenger is not involved.
Because the eCBs are hydrophobic molecules that might aggregate, it is possible that eCBs move across the synapse as part of a lipid-binding protein-delivery complex. Interestingly, it has been suggested that eCB uptake involves caveolae-related endocytosis (45), and caveolin-1, which is a marker for caveolae, displays properties similar to a fatty acid-binding protein (46). Because AEA-induced depression requires synaptic activity, but does not appear to require receptor or channel activation, it is possible that paired-pulse stimulation simply affects the physical state of the cell plasma membrane lipid bilayer in a way that modulates the transmembrane transport, which was previously reported for carrier-mediated transport of 5-hydroxytryptamine (47). The molecular mechanisms linking afferent activation to the activity of the AMT require further study, and a more detailed analysis of AMT function and incorporation into the cell membrane is required to fully understand the role of afferent activation for this transporter.
Is Afferent Activation Involved in eCB Release or CB1 Receptor Activation?
AEA-induced depression of EPSC amplitude depended on afferent activation, whereas WIN 55,212-2-induced depression occurred independently of such activation. This finding indicates that the role for afferent activation in these experiments is related to mobilization and/or release of eCBs and is probably linked to activation of a postsynaptic eCB transporter or an intrasynaptic carrier, rather than presynaptic CB1-mediated mechanisms. A new study by Malenka and coworkers (32) showed that afferent activation is required for induction of LTD mediated by a CB1R agonist. If afferent activation is required for both pre- and postsynaptic events, the synapses at which eCB-LTD occur would be strictly regulated. If activation of the AMT also requires strong synaptic activity in vivo, our data suggest that no depression would occur at inactive synapses, and eCB release and LTD would only be induced at highly activated synapses. These findings also might explain why it is difficult to evoke depolarization-induced supression of excitation at striatal synapses, whereas short-term depression and eCB-dependent LTD are readily induced.
AEA-Induced Depression at Inhibitory Synapses.
AEA-induced depression at inhibitory synapses was not dependent on afferent activation to the same extent as excitatory synapses. No differences were seen between paired- and single-pulse stimulation protocols, and a slight depression was induced even when stimulation was suspended. However, in contrast to direct CB1R activation by explicit application of a synthetic agonist, the depression magnitude was increased after 10 min of additional stimulation, suggesting that, although eCB signaling can occur, it is enhanced considerably by afferent activation. This idea is further supported by our finding that the sIPSC frequency, which was depressed after 20 min of recording without stimulation, was further reduced by afferent activation. This observation shows that the AEA-induced depression had not reached a maximum level within the 20-min time frame in the absence of afferent activation.
It is possible that the difference between excitatory and inhibitory synapses lies in the number of presynaptic CB1R, which are highly expressed at inhibitory synapses (48). If AMT activity is regulated by the level of synaptic activation, a small amount of eCBs might be released in the absence of stimulation, whereas a large amount is released during strong afferent activation. Based on the high concentration of CB1R at striatal inhibitory synapses, a low concentration of eCBs and, correspondingly, a low level of synaptic activity might be required for the induction of depression at these synapses. These factors also may underlie the lack of CB1R antagonist reversal seen at inhibitory synapses. If the number of activated CB1R is high, it is possible that the antagonist wash-on time that we used might be too short to allow us to detect reversal of inhibition. Unfortunately, it is difficult to maintain whole-cell recordings long enough to determine whether inhibition of IPSCs would eventually reverse. It also is possible that the requirements for LTD formation vary between different synapses.
Materials and Methods
Coronal brain slices containing the striatum were prepared from p15–20 rats as described in ref. 9. Slices were allowed to equilibrate for at least 1 h in aCSF (124 mM NaCl, 4.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 26 mM NaHCO3, 1.2 mM NaH2PO4, and 10 mM d-glucose) continuously bubbled with a mixture of 95% O2/5% CO2 gas. One hemisphere of a slice was transferred to a recording chamber, and a slice electrophysiological system was set up as described in ref. 9. Internal solutions consisted of 120 mM CsMeSO3, 5 mM NaCl, 10 mM TEA-Cl, 10 mM Hepes, 5 mM QX-314, 1.1 mM EGTA, 4 mM Mg-ATP, and 0.3 mM Na-GTP for most experiments examining EPSCs or 150 mM CsCl, 10 mM Hepes, 2 mM MgCl2, 0.3 mM Na-GTP, 3 mM Mg-ATP, 0.2 mM BAPTA for all experiments examining IPSCs and one experiment examining EPSCs. The pH was set at 7.2 with CsOH, and osmolarity was set to 300 mmol/kg with sucrose. For IPSC measurements, 5 μM 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX) and 50 μM 5 DL-2-Amino-5 phosphonovaleric acid (AP-5) was added to the aCSF.
Currents were measured in conventional ruptured-patch whole-cell mode in MSNs voltage-clamped at −50 or −70 mV. The holding potential of −50 mV was chosen so that these studies could be reliably compared with published data from our previous experiments examining LTD induced by calcium channel activation (9). Baseline synaptic currents were evoked by single or paired (50-msec interpulse interval) stimuli delivered every 20 sec through a bipolar electrode placed in the overlying white matter. Stimulus parameters were adjusted to elicit baseline EPSC or IPSC amplitudes between 200 and 400 pA. Once a stable baseline was achieved, the amplitude of the EPSC or IPSC was compared with EPSC or IPSC amplitude 20–25 min after stabilization of the baseline and presented as mean value ± 95% confidence interval. EPSC and IPSC data in time-course figures are plotted as mean amplitude compared with baseline with SEM. The paired t test was used for statistical analysis.
Loading of 50 μM eCBs AEA or 2-AG was used to evaluate eCB release and subsequent presynaptic depression at excitatory and inhibitory synapses as in previous studies (2, 30). Recordings were initiated immediately upon establishing the whole-cell recording configuration, and we did not try to avoid AEA spill-over after loading. For estimation of PPR, mean EPSC/IPSC 2 amplitude during a 5-min recording period over mean EPSC/IPSC 1 amplitude during the same time frame was calculated.
In a subset of recordings, sIPSCs were recorded in AEA-loaded cells 5, 10, 15, and 20 min after establishing the whole-cell recording and after a 10-min afferent stimulation with a paired-pulse protocol. sIPSCs were analyzed by using the Mini-Analysis program version 6.0.3 (Synaptosoft).
Most chemicals were purchased from Sigma–Aldrich or Tocris. AEA was dissolved in ethanol to 25 mM, and 2-AG was received from the manufacturer dissolved in 10 mg/ml acetonitrile solution. Both eCBs were used at 50 μM, and thus final vehicle concentrations were 0.2% EtOH and 0.19% acetonitrile. NBQX was dissolved in H2O to 50 mM and used at 5 μM, and AP-5 was diluted in aCSF to 50 μM. VDM11 was diluted from 50 mM DMSO stock solutions and used at 10 μM, whereas UCM707 was diluted in ethanol to 50 mM and used at 10 μM. MPEP and CPCCOEt were dissolved in DMSO to 100 mM and used at 40 μM MPEP and 80 μM CPCCOEt. The l-NAME was dissolved in aCSF to 100 μM and kept on ice during the course of the day. Hemoglobin from rat was diluted in aCSF to 500 mg/liter. Nifedipine was dissolved in DMSO and used at a 20 μM final concentration. AM251 and WIN 55,212-2 were dissolved in DMSO to 50 mM and 10 mM, respectively, and stock solutions were diluted in aCSF containing 0.5 g/liter BSA and used at 3 μM AM251 and 1 μM WIN 55,212-2. Experiments were performed at 30–33°C and discontinued if the series resistance varied by >20% or increased >30 MΩ.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Bradley E. Alger for his suggestions. This work was supported by the Division of Intramural Clinical and Basic Research, National Institute on Alcohol Abuse and Alcoholism/National Institutes of Health, and the Swedish Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706873104/DC1.
References
- 1.Gerdeman G, Lovinger DM. J Neurophysiol. 2001;85:468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- 2.Ronesi J, Gerdeman GL, Lovinger DM. J Neurosci. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chevaleyre V, Takahashi KA, Castillo PE. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- 4.Alger BE, Pitler TA, Wagner JJ, Martin LA, Morishita W, Kirov SA, Lenz RA. J Physiol. 1996;496:197–209. doi: 10.1113/jphysiol.1996.sp021677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitler TA, Alger BE. Neuron. 1994;13:1447–1455. doi: 10.1016/0896-6273(94)90430-8. [DOI] [PubMed] [Google Scholar]
- 6.Melis M, Perra S, Muntoni AL, Pillolla G, Lutz B, Marsicano G, Di Marzo V, Gessa GL, Pistis M. J Neurosci. 2004;24:10707–10715. doi: 10.1523/JNEUROSCI.3502-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 8.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 9.Adermark L, Lovinger DM. J Neurosci. 2007;27:6781–6787. doi: 10.1523/JNEUROSCI.0280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, Purpura DP, Castillo PE. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerdeman GL, Lovinger DM. Br J Pharmacol. 2003;140:781–789. doi: 10.1038/sj.bjp.0705466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alger BE. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- 13.Piomelli D. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Wang L, Harvey-White J, Huang BX, Kim HY, Luquet S, Palmiter RD, Krystal G, Rai R, Mahadevan A, et al. Neuropharmacology. 2008;54:1–7. doi: 10.1016/j.neuropharm.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bender VA, Bender KJ, Brasier DJ, Feldman DE. J Neurosci. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maccarrone M, Fiori A, Bari M, Granata F, Gasperi V, De Stefano ME, Finazzi-Agro A, Strom R. Thromb Haemost. 2006;95:117–127. [PubMed] [Google Scholar]
- 17.Maccarrone M, Valverde O, Barbaccia ML, Castane A, Maldonado R, Ledent C, Parmentier M, Finazzi-Agro A. Eur J Neurosci. 2002;15:1178–1186. doi: 10.1046/j.1460-9568.2002.01957.x. [DOI] [PubMed] [Google Scholar]
- 18.Hillard CJ, Jarrahian A. Chem Phys Lipids. 2000;108:123–134. doi: 10.1016/s0009-3084(00)00191-2. [DOI] [PubMed] [Google Scholar]
- 19.Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, Deutsch DG. Proc Natl Acad Sci USA. 2003;100:4269–4274. doi: 10.1073/pnas.0730816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glaser ST, Kaczocha M, Deutsch DG. Life Sci. 2005;77:1584–1604. doi: 10.1016/j.lfs.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Beltramo M, Piomelli D. Neuroreport. 2000;11:1231–1235. doi: 10.1097/00001756-200004270-00018. [DOI] [PubMed] [Google Scholar]
- 22.Piomelli D, Beltramo M, Glasnapp S, Lin SY, Goutopoulos A, Xie XQ, Makriyannis A. Proc Natl Acad Sci USA. 1999;96:5802–5807. doi: 10.1073/pnas.96.10.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Petrocellis L, Bisogno T, Maccarrone M, Davis JB, Finazzi-Agro A, Di Marzo V. J Biol Chem. 2001;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- 24.Pillolla G, Melis M, Perra S, Muntoni AL, Gessa GL, Pistis M. Psychopharmacology (Berl) 2007;191:843–853. doi: 10.1007/s00213-007-0733-z. [DOI] [PubMed] [Google Scholar]
- 25.Ligresti A, Morera E, Van Der Stelt M, Monory K, Lutz B, Ortar G, Di Marzo V. Biochem J. 2004;380:265–272. doi: 10.1042/BJ20031812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hillard CJ, Shi L, Tuniki VR, Falck JR, Campbell WB. J Mol Neurosci. 2007;33:18–24. doi: 10.1007/s12031-007-0045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dittman JS, Kreitzer AC, Regehr WG. J Neurosci. 2000;20:1374–1385. doi: 10.1523/JNEUROSCI.20-04-01374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster KA, Kreitzer AC, Regehr WG. Neuron. 2002;36:1115–1126. doi: 10.1016/s0896-6273(02)01106-6. [DOI] [PubMed] [Google Scholar]
- 29.Safo PK, Regehr WG. Neuron. 2005;48:647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Gerdeman GL, Ronesi J, Lovinger DM. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- 31.Zhuang SY, Bridges D, Grigorenko E, McCloud S, Boon A, Hampson RE, Deadwyler SA. Neuropharmacology. 2005;48:1086–1096. doi: 10.1016/j.neuropharm.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Singla S, Kreitzer AC, Malenka RC. J Neurosci. 2007;27:5260–5264. doi: 10.1523/JNEUROSCI.0018-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sjostrom PJ, Turrigiano GG, Nelson SB. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 34.Kreitzer AC, Regehr WG. J Neurosci. 2000;20:1348–1357. doi: 10.1523/JNEUROSCI.20-04-01348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin HH, Davis MI, Ronesi JA, Lovinger DM. J Neurosci. 2006;26:11811–11820. doi: 10.1523/JNEUROSCI.3196-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreitzer AC, Malenka RC. J Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. J Neurosci. 1992;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calabresi P, Gubellini P, Centonze D, Sancesario G, Morello M, Giorgi M, Pisani A, Bernardi G. J Neurosci. 1999;19:2489–2499. doi: 10.1523/JNEUROSCI.19-07-02489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centonze D, Gubellini P, Pisani A, Bernardi G, Calabresi P. Rev Neurosci. 2003;14:207–216. doi: 10.1515/revneuro.2003.14.3.207. [DOI] [PubMed] [Google Scholar]
- 40.Maccarrone M, Bari M, Lorenzon T, Bisogno T, Di Marzo V, Finazzi-Agro A. J Biol Chem. 2000;275:13484–13492. doi: 10.1074/jbc.275.18.13484. [DOI] [PubMed] [Google Scholar]
- 41.Isokawa M, Alger BE. J Neurophysiol. 2006;95:3001–3011. doi: 10.1152/jn.00975.2005. [DOI] [PubMed] [Google Scholar]
- 42.Maccarrone M, Bari M, Battista N, Finazzi-Agro A. Blood. 2002;100:4040–4048. doi: 10.1182/blood-2002-05-1444. [DOI] [PubMed] [Google Scholar]
- 43.van der Stelt M, Trevisani M, Vellani V, De Petrocellis L, Schiano Moriello A, Campi B, McNaughton P, Geppetti P, Di Marzo V. EMBO J. 2005;24:3026–3037. doi: 10.1038/sj.emboj.7600784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. J Neurosci. 2005;25:6826–6835. doi: 10.1523/JNEUROSCI.0945-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McFarland MJ, Porter AC, Rakhshan FR, Rawat DS, Gibbs RA, Barker EL. J Biol Chem. 2004;279:41991–41997. doi: 10.1074/jbc.M407250200. [DOI] [PubMed] [Google Scholar]
- 46.Stremmel W, Pohl L, Ring A, Herrmann T. Lipids. 2001;36:981–989. doi: 10.1007/s11745-001-0809-2. [DOI] [PubMed] [Google Scholar]
- 47.Block ER, Edwards D. Am J Physiol. 1987;253:C672–C678. doi: 10.1152/ajpcell.1987.253.5.C672. [DOI] [PubMed] [Google Scholar]
- 48.Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.