Abstract
BH3-only proteins couple diverse stress signals to the evolutionarily conserved mitochondrial apoptosis pathway. Previously, we reported that the activation of the BH3-only protein p53-up-regulated mediator of apoptosis (Puma) was necessary and sufficient for endoplasmic reticulum (ER) stress- and proteasome inhibition-induced apoptosis in neuroblastoma and other cancer cells. Defects in protein quality control have also been suggested to be a key event in ALS, a fatal neurodegenerative condition characterized by motoneuron degeneration. Using the SOD1G93A mouse model as well as human post mortem samples from ALS patients, we show evidence for increased ER stress and defects in protein degradation in motoneurons during disease progression. Before symptom onset, we detected a significant up-regulation of Puma in motoneurons of SOD1G93A mice. Genetic deletion of puma significantly improved motoneuron survival and delayed disease onset and motor dysfunction in SOD1G93A mice. However, it had no significant effect on lifespan, suggesting that other ER stress-related cell-death proteins or other factors, such as excitotoxicity, necrosis, or inflammatory injury, may contribute at later disease stages. Indeed, further experiments using cultured motoneurons revealed that genetic deletion of puma protected motoneurons against ER stress-induced apoptosis but showed no effect against excitotoxic injury. These findings demonstrate that a single BH3-only protein, the ER stress-associated protein Puma, plays an important role during the early stages of chronic neurodegeneration in vivo.
Keywords: neurodegeneration, SOD1, Bcl-2 family
ALS is a fatal neurodegenerative condition characterized by progressive loss of motoneurons from the spinal cord, brainstem, and motor cortex (1). Although most cases of ALS are sporadic, 5–10% of cases are familial (fALS). Mutations in the gene encoding Cu/Zn super oxide dismutase (SOD1) have been found in 20–25% of fALS cases (2). Transgenic mice overexpressing human mutant SOD1 develop an ALS-like phenotype (3) of progressive motoneuron degeneration with a reduced lifespan.
Although the precise etiology of ALS remains unclear, the common sequestration of aberrant proteins into inclusions in motoneurons and astrocytes in ALS patients (4) and SOD1G93A mice (5) implicate protein misfolding and aggregation as common aspects of pathogenesis. In SOD1G93A mice, misfolded mutant SOD1 has been shown to accumulate in the endoplasmic reticulum (ER) (6, 7). In motoneurons of ALS patients and SOD1G93A mice, inclusions also often contain ubiquitinylated proteins and protein chaperones (8), suggesting that accumulation of misfolded proteins also disturbs protein degradation and sequesters cytoplasmic chaperones that are required for catalyzing the folding/refolding of proteins.
When misfolded or unfolded proteins accumulate in the lumen of the ER, it results in a condition denoted “ER stress response” or “unfolded protein response” (UPR). The UPR provokes a transcriptional response, which triggers increased expression of chaperones to promote the correct folding of nascent proteins in the ER. However, if accumulation of unfolded proteins persists, ER stress can also activate apoptotic cell death pathways (9). We have previously shown that the proapoptotic BH3-only protein Puma was necessary and sufficient for ER stress-induced apoptosis (10) and was also activated in cells subjected to proteasome inhibition (11). BH3-only proteins constitute a proapoptotic subgroup of the Bcl-2 family that share a short (9–16 aa) Bcl-2 homology (BH) 3 region. This domain is required for their ability to bind and neutralize Bcl-2-like prosurvival proteins, leading to the activation of Bax and Bak and to the caspase cascade (12).
Eight mammalian BH3-only proteins have been identified to date. Although redundancies exist, it is believed that individual BH3-only proteins couple specific stress stimuli to the activation of the mitochondrial apoptosis pathway (13). In the present study, we tested the hypothesis that the activation of a single BH3-only protein is sufficient to mediate chronic neurodegeneration in vivo by using SOD1G93A mice.
Results
Evidence of ER Stress and Protein Degradation Defects in SOD1G93A Mice and Sporadic ALS Patients.
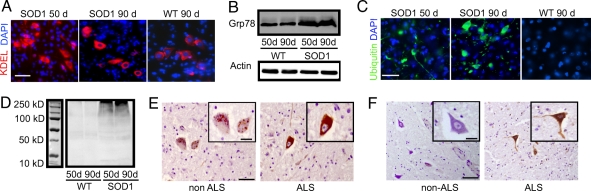
In SOD1G93A mice, motoneuron degeneration in the lumbar spinal cord is histologically detectable from 60 days, and overt disease symptoms such as decreased stride length and tremor of the hind limbs are evident from 90 days (3, 14, 15). We investigated the activation of target genes of the UPR in SOD1G93A mice. The UPR target genes include the ER-resident chaperones, Grp78 and Grp94, which help to alleviate ER stress by promoting the correct folding of proteins in the ER. Using an anti-KDEL antibody, which recognizes both Grp78 and Grp94, we observed that the expression of ER-resident chaperones in motoneurons of SOD1G93A mice increased as early as 50 days compared with WT littermates (Fig. 1A). An increase in the expression of Grp78 was confirmed by Western blotting (Fig. 1B). In SOD1G93A mice, we also detected an increase in ubiquitin immunostaining in motoneurons during the early stage of disease progression, and this result was also confirmed by Western blotting (Fig. 1 C and D). The increase in ubiquitin expression in motoneurons of SOD1G93A mice is suggestive of an increased accumulation of aberrant, nondegradable proteins or of direct defects in protein degradation. Together, these results confirmed previous reports of evidence for ER stress and defects in protein degradation in SOD1G93A mice (6–8).
Fig. 1.
Protein degradation defects in SOD1G93A mice and ALS patients. (A) Immunocytochemistry in spinal cord sections for KDEL, which recognizes both Grp78 and Grp94. (Scale bar: 75 μm.) (B) Western blot for Grp78 expression. (C) Immunocytochemistry in spinal cord cross-sections for ubiquitin. (Scale bar: 100 μm.) (D) Western blot for ubiquitin expression with molecular mass marker. (E and F) Post mortem spinal cord cross-sections showing anterior horn motoneurons immunostained with antibodies to KDEL (brown) (E) or ubiquitin (brown) (F) both counterstained with haematoxylin (blue). (Scale bars: E, 0.5 mm; F, 0.3 mm)
Post mortem spinal cord cross sections from sporadic ALS (sALS) and non-ALS patients were also immunostained with antibodies to KDEL and ubiquitin [Fig. 1 E and F; see supporting information (SI) Table 1 for case details]. Anterior horn motoneurons from both non-ALS and sALS cases stained positively for KDEL; however, in sALS cases, a greater proportion of neurons showed strong expression of KDEL compared with non-ALS cases (P < 0.05, SI Table 2). Similarly, the intensity of ubiquitin immunostaining was greater in sALS cases compared with non-ALS cases (SI Table 2). These results indicate that motoneurons that have not yet degenerated in ALS patients show evidence of ER stress and defects in protein degradation.
Puma Is Activated in Motoneurons During Disease Progression.
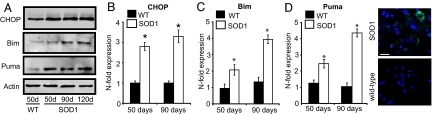
C/EBP homologous protein (CHOP/GADD153) is a transcription factor induced by ER stress and other stressors that has been suggested to be involved in the transition of ER-stress responses from prosurvival to proapoptotic (16–18). We detected a moderate increase in C/EBP homologous protein (CHOP) mRNA and protein expression in ventral horn tissue homogenates from the spinal cord of SOD1G93A mice from 50 days (Fig. 2 A and B). We also detected a moderate increase in the expression of Bim, a BH3-only protein and a CHOP target gene (18) (Fig. 2 A and C). Interestingly, we also detected a pronounced and progressive increase in the expression of Puma (Fig. 2 A and D). Immunostaining for Puma in spinal cord sections showed Puma expression directly in motoneurons at an early stage in disease progression (70 days) in SOD1G93A mice (Fig. 2E). These findings suggested that Puma may be involved in controlling motoneuron degeneration in vivo.
Fig. 2.
ER-stress in SOD1G93A mice. (A) Western blot analysis of CHOP, Puma, and Bim expression. (B–D) Real-time quantitative PCR analysis of CHOP mRNA (B), Bim mRNA (C), and Puma mRNA (D). n = 3. Error bars are ± SEM. (E) Immunocytochemistry in spinal cord sections at 70 days for Puma (green) and the nuclear stain DAPI (blue). (Scale bar: 50 μm.)
Genetic Deletion of puma in SOD1G93A Mice Delays Disease Progression.
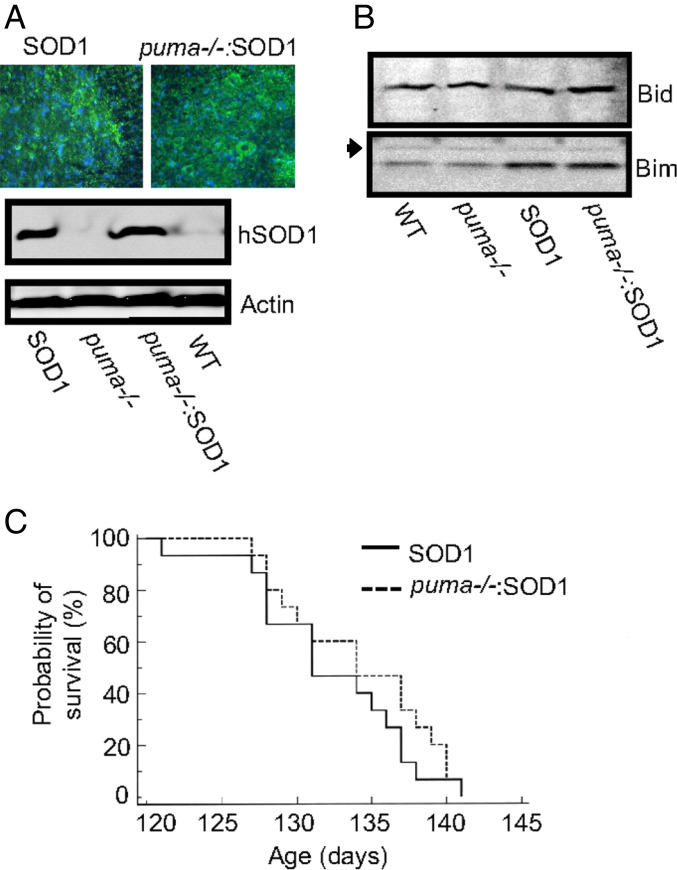
In light of previous reports showing protective effects of genetic deletion of puma in models of ER stress- and proteasome inhibition-induced apoptosis (10, 11, 19, 20), we next tested whether Puma is involved in motoneuron degeneration of SOD1G93A mice. A cross-breeding program was carried out to generate SOD1G93A mice deficient for puma. Cross-breeding of SOD1G93A mice with puma−/− mice did not alter the expression of human SOD1 (Fig. 3A) or lead to any compensatory changes in other BH3-only proteins (Bim and Bid) (Fig. 3B).
Fig. 3.
Lifespan analysis in cross-bred mice. (A) Immunocytochemistry on spinal cord sections for human SOD1 (green) and the nuclear stain DAPI (blue). (Scale bar: 100 μm.) Western blot analysis of human SOD1 expression in cross-bred mice. (B) Western blot analysis of Bim and Bid expression in cross-bred mice. n.s. indicates a nonspecific band showing equal sample loading. (C) Kaplan–Meyer analysis of survival.
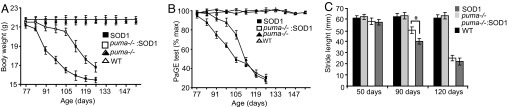
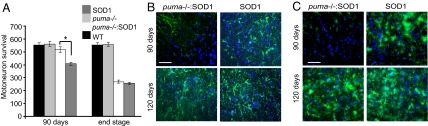
Analysis of the lifespan of cross-bred mice demonstrated that deletion of puma in SOD1G93A mice did not significantly improve lifespan (Fig. 3C). However, further analysis showed a significant delay in disease progression in cross-bred mice. Disease progression was monitored using functional assessments of motor performance. Analysis of animal weight showed that, from 77 days, the weight of SOD1G93A mice steadily declined (Fig. 4A). However, in puma−/−;SOD1G93A mice, body weight did not begin to decrease significantly until 105 days. The paw-grip endurange (PaGE) test was used to monitor the onset and progression of motor symptoms in cross-bred mice (Fig. 4B). The PaGE test performance showed that, from 77 days, the performance of SOD1G93A mice began to steadily decline. This decline in PaGE test performance was delayed in puma−/−;SOD1G93A mice and only became evident at 98 days. Because the PaGE test is a sensitive assessment of detecting early motor signs in SOD1G93A mice (21), these results indicated that genetic deletion of puma delayed the onset of motor deficits in SOD1G93A mice.
Fig. 4.
Assessment of disease progression. (A) Body weight of mice during disease progression. (B) Analysis of PaGE test performance during disease progression (expressed as a percentage of maximum performance at 10 weeks). (C) Stride-length analysis. (All error bars are ± SEM. n = 10–15 in each group. *, P ≤ 0.05).
To quantify motor performance, footprint analysis of stride length was performed (Fig. 4C) and showed significant impairment in walking patterns of SOD1G93A mice at 90 days, when stride length was reduced to 40 mm (± 2.7) compared with 62 mm (± 2.9) in WT mice (P ≤ 0.005). In puma−/−;SOD1G93A, mice stride length at 90 days was 50 mm (± 3.1, P ≤ 0.005). By 120 days, stride length in SOD1G93A mice was only 22 mm (± 3.2), but puma−/−;SOD1G93A failed to show a significant improvement in stride length at this late stage in the disease.
puma Deficiency Protects Against Motoneuron Loss at the Early Stage of Disease.
We next examined whether puma deficiency delayed motoneuron degeneration by using histological analysis of sciatic motoneuron survival in the lumbar spinal cord (Fig. 5A). In SOD1G93A mice at 90 days, there was a significant decrease in sciatic motoneuron survival, with only 423 (± 12.7) motoneurons surviving compared with 573 (± 19.1) surviving in WT mice. At 90 days, sciatic motoneuron survival of puma−/−;SOD1G93A mice was significantly increased compared with SOD1G93A littermates, such that 537 (± 22.6) motoneurons survived. Motoneuron integrity was also assessed by immunostaining sections with antibodies to choline acetyltransferase (Chat) (22). The number of Chat-positive motoneurons counted in the sciatic motor pool of mice at 90 days was not significantly different from the number of gallocyanin-stained motoneurons counted in the same animals (SI Fig. 7).
Fig. 5.
Motoneuron survival and histology. (A) Motoneuron survival in the sciatic motor pool. n = 7–9. *, P ≤ 0.05. (B and C) Immunocytochemistry in lumbar spinal cord cross-sections for the astrocyte marker GFAP (green) and the nuclar stain DAPI (blue) (B) and the microglial marker CD11b (green) and the nuclar stain DAPI (blue) (C). (Scale bars, 150 μm.)
The improvement in motoneuron survival at 90 days in puma−/−;SOD1G93A mice suggested that the delay in disease onset and disease progression was a direct consequence of increased motoneuron survival. However, the significant increase in motoneuron survival in puma−/−;SOD1G93A mice at 90 days was not maintained at the disease end point, and only 278 (± 11.2) motoneurons survived (Fig. 5A). This finding was not significantly different from motoneuron survival in SOD1G93A mice, in which 264 (± 10.4) motoneurons survived (P ≥ 0.05).
We further examined the spinal cords of puma−/−;SOD1G93A mice at 90 days and at the end stage to determine whether the delay in motoneuron degeneration was accompanied by a delay in gliosis and/or microglial activation. A reduction in the rate of apoptosis could also induce a shift to necrotic cell death pathways and a subsequent increase in tissue inflammation. Interestingly, we found that, at 90 days, both gliosis and microglial activation were reduced rather than increased in puma−/−;SOD1G93A mice compared with that in SOD1G93A mice (Fig. 5 B and C). By the disease end stage, there was no difference in the level of immunostaining for glial fibrillary acidic protein (GFAP), a marker for astrocyte activation, or CD11b, a marker for microglial activation, between puma−/−;SOD1G93A mice and SOD1G93A mice.
Genetic Deletion of puma Protects Cultured Motoneurons Against ER Stress-Induced Apoptosis but Has No Protective Effect Against Excitotoxic Injury.
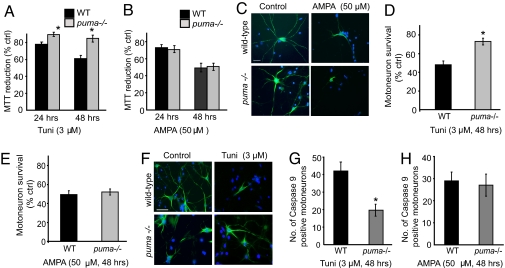
ALS is multifactorial disease in which stress signals other than defects in protein quality control are likely to be involved. Excitotoxicity due to defective glutamate signaling has been particularly implicated in ALS pathogenesis (23, 24). Here we wished to determine whether or not puma also contributed to the death of motoneurons induced by other stress paradigms, in particular excitotoxic injury. Primary motoneuron cultures prepared from puma−/− and WT mouse embryos were exposed to the ER-stress inducer tunicamycin or the glutamate agonist AMPA at 10 days in vitro (DIV). Because motoneuron cultures consist largerly of nonproliferating cells, overall toxicity was analyzed by determining the mitochondrial redox status by using a 3-(4,5-dimethylthiazol-2-yl)-2,5-dphenyltetrazolium bromide (MTT) assay. Exposure of WT primary motoneuron cultures to 3 μM tunicamycin for 48 h resulted in a significant decrease in MTT reduction to 61% (± 3.4). However, in puma−/−, motoneuron cultures MTT reduction was significantly improved to 85% (± 3.6, Fig. 6A). Exposure of WT motoneurons to the glutamate agonist α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) (50 μM, 48 h) also resulted in a similar decrease in MTT reduction. However, puma−/− motoneurons failed to be protected from AMPA-mediated excitotoxic injury (Fig. 6 B and C).
Fig. 6.
Puma−/− motoneurons in vitro. (A) MTT reduction after tunicamycin (tuni) treatment. n = 3. (B) MTT reduction after AMPA treatment (50 μM). n = 3. (C) Photomicrograhps of SMI-32- (green) and DAPI- (blue) immunostained primary motoneuron cultures after AMPA treatment. (Scale bar: 75 μm.) (D and E) Direct counts of motoneuron survival after tunicamycin (D) and AMPA (E) treatment. n = 3. (F) Photomicrograhps of SMI-32- (green) and DAPI- (blue) immunostained primary motoneuron cultures after tunicamycin treatment. (Scale bar: 100 μm.) (G and H) Direct counts of caspase 9-positive motoneurons in cultures after tunicamycin (G) and AMPA (H) treatment. All Error bars are ± SEM. *, P ≤ 0.05.
We also performed direct counts of motoneuron survival by using the trypan blue exclusion method in cultures immunolabeled with an antibody to SMI-32 (Fig. 6 C–E). After exposure to tunicamycin (3 μM, 48 h), only 48% (± 4.1, Fig. 6D) of WT motoneurons survived. In contrast, 73% (± 3.7) of puma−/− motoneurons survived (P < 0.05). However, there was no significant difference in motoneuron survival between WT and puma−/− mice motoneuron cultures after an exposure to AMPA (50 μM, 48 h) (P ≥ 0.05, Fig. 6E), suggesting that puma does not protect motoneurons against excitotoxic injury.
We finally investigated whether the mitochondrial apoptosis pathway initiated by BH3-only proteins was inhibited during tunicamycin-induced apoptosis in puma−/− motoneurons. Immunostaining of active caspase-9 revealed potent inhibition of caspase-9 activation during ER stress, but not AMPA-mediated excitotoxic injury in the puma−/− motoneurons compared with WT motoneurons (Fig. 5 G and H).
Discussion
Here we provide evidence for stress responses in motoneurons of SOD1G93A mice and sALS patients, originating from defects in protein quality control. These responses include increased expression of ER-resident chaperones, indicative of ER stress, as well as pronounced defects in protein degradation. In SOD1G93A mice, both occurred before the onset of symptoms. These results confirm previous reports of ER stress in SOD1G93A mice, where accumulation of misfolded mutant SOD1 in the ER (6), an increase in ER-resident chaperone expression (7), and activation of UPR transcription factors (6) have been reported. Prolonged ER stress is also associated with activation of the ER-associated degradation (ERAD) pathway (25, 26), which targets misfolded proteins for degradation via the proteasome. The pronounced increase in ubiquitin expression in motoneurons in SOD1G93A mice occurring in parallel with increased ER stress suggests that the proteasome may be overloaded with misfolded proteins and, hence, may be dysfunctional (8, 27), although these may also be independent events. We also provide evidence for an increased expression of CHOP, a transcription factor believed to mediate proapoptotic responses and that is induced by ER stress and proteasome inhibition but also by other stress stimuli (28, 29). CHOP has been implicated in mediating ER stress-induced apoptosis via activation of the BH3-only protein Bim (18) or by death receptor 5 expression (30). However, in this study we also found a pronounced and early increase in Puma expression.
Our data demonstrate that genetic deletion of puma protected motoneurons from degeneration in the SOD1G93A mouse model, delayed disease onset, and lead to an improved motor performance. However, deletion of puma did not result in a significant increase in lifespan. The findings of this study therefore support the concept of a critical role for puma during the early stage of chronic neurodegeneration in ALS. puma has been described as a p53 target gene (31, 32); however, puma induction during ER stress and other death stimuli can also occur in a p53-independent manner (10, 33). In this context, it is interesting to note that previous studies have shown that SOD1G93A mice cross-bred with p53 knockout mice showed no difference in disease onset or motoneuron degeneration compared with SOD1G93A mice (34, 35). In further studies using cultured motoneurons, we found that deletion of puma protected motoneurons from ER stress-induced cell death, but no improvement was seen against excitotoxic cell death, which has also been implicated in ALS (1, 23, 24).
Although we clearly demonstrate that the knockdown of a single proapoptotic BH3-only protein, puma, is sufficient to delay motoneuron degeneration and disease progression in SOD1G93A mice, at later stages Puma-independent cell death pathways and/or other BH3-only proteins may participate in disease progression and may substitute for puma loss. These BH3-only proteins include Bim and Bid, which, like Puma, have the capacity to inactivate all prosurvival Bcl-2 family proteins (36). Bim can be activated via CHOP during ER stress (18), as well as via JNK and p38 kinase pathways (37). Activation of both p38 MAPK and JNK has been shown to occur in activated microglial cells of SOD1G93A mice at an advanced stage of the disease (38). It has recently been shown that bim deficiency in SOD1G93A mice also reduces cellular apoptosis in the spinal cord of SOD1G93A mice (39). In this context, it would be interesting to examine the effect of a simultaneous deletion of bim and puma in SOD1G93A mice.
Bid is involved in the so-called extrinsic apoptosis pathway. This pathway is activated by death receptors of the TNF receptor superfamily. It involves activation of the FADD/caspase-8 cascade and cleavage of Bid by caspase-8 into a truncated, active form (tBid) (40). In SOD1G93A mice, there is evidence for the involvement of the extrinsic pathway in motoneuron degeneration (41, 42), including the detection of the cleaved form of Bid and active caspase 8 in SOD1G93A mice. Because activated caspase 8 has been detected in the late stages of disease progression, Bid may be a potential substitute for Puma or Bim at later stages in disease progression, when proinflammatory responses become more prominent.
A recent study has examined the effect of genetically deleting bax in SOD1G93A mice (43). Bax is central in activating the mitochondrial apoptosis pathway in neurons, as Bax activation directly induces mitochondrial outer membrane permeabilization and the release of caspase-activating factors. Bax deletion in SOD1G93A mice delayed disease onset and moderately increased lifespan but ultimately failed to alter disease duration. The protective effect of bax deletion in SOD1G93A mice was attributed to retarding denervation at the neuromuscular junction (43). Other studies have shown that therapeutic strategies aimed at targeting components of the (mitochondrial) apoptotic pathway, including downstream caspases, can delay disease progression and to some extent improve lifespan (44). Although the results of these findings and the present study support a role of the BH3-only protein/Bax/caspase pathway in motoneuron degeneration in SOD1G93A mice, approaches to block these pathways cannot abrogate it. Indeed, at later stages of disease, alternative and/or necrotic cell-death pathways may be triggered by pathological events such as mitochondrial dysfunction and ATP depletion, inflammation, or astrocyte-mediated toxicity. However, the results of our study also suggest that strategies that inhibit apoptosis pathways may not necessarily accelerate the induction of proinflammatory processes (Fig. 5 B and C).
In summary, we show that the BH3-only protein Puma is critically involved in disease progression in SOD1G93A mice, suggesting that Puma may be a critical factor in controlling chronic neurodegeneration in ALS and other neurodegenerative disorders that involve defects in protein quality control.
Materials and Methods
Immunohistochemistry.
Spinal cord sections were incubated with anti-KDEL antibody (1:500; Stressgen Biotechnology), anti-Ubiquitin antibody (1:500; Biomol), anti-Puma antibody (1:250; Abcam), anti-GFAP antibody (1:500; DAKO), anti-CD11b antibody (1:500; Abcam), or anti-ChAT antibody (1:500; Abcam) to detect respective proteins, as described in ref. 15. Human spinal cord sections were deparaffinized in xylene, and antigen retrieval was performed using citrate buffer and incubated with primary antibody (KDEL, 1:250 as per above; ubiquitin, 1:200 as per above), biotinylated secondary antibody (1:500; Jackson Immuno Research), and visualized using Sigma fast 3,3′-diaminobenzadine tables.
Semiquantitative Analysis of Immunohistochemistry.
The intensity of immunostaining in human spinal cord sections was assessed using a four-point scale (0, staining absent; +, weak; ++, moderate; +++, strong) (45). Sections from 11 ALS cases and 10 non-ALS cases were assessed (post mortem interval, 10–14 h). Immunostaining was considered weak (+) if it was poorly apparent at low-power magnification but was identifiable using the high-power objective. Moderate (++) and strong (+++) staining reactions were apparent at low power. Five fields (area = 0.125 mm2) were assessed in each anterior horn. The number of motoneurons in each staining category was then expressed as a proportion of the total number of motoneurons counted (45). Statistical analysis was assessed using the Fisher's exact test.
Western Blotting.
Preparation of cell lysates, Western blotting and immunodetection was performed as described in ref. 10. The blots were incubated with anti-Grp78 antibody (1:1,000; Santa Cruz Biotechnologies), anti-human SOD1 antibody (1:500; Sigma–Aldrich), anti-CHOP (GADD153) antibody (1:500; Santa Cruz Biotechnology), anti-Puma antibody (1:500; Abcam), anti-Ubiquitin antibody (1:500; Biomol), anti-Bim antibody (1:2,000; Santa Cruz Biotechnology), anti-Bid antibody (1:1,000; Santa Cruz Biotechnology), or an anti-β-actin antibody (1:3,000; Sigma–Aldrich).
Real-Time Quantitative PCR.
Total RNA was extracted using the RNeasy Mini Kit (Qiagen). First-strand cDNA synthesis was performed according to the manufacturers' instruction by using 2 μg of Moloney murine leukaemia virus reverse transcriptase (Invitrogen). Quantitative real-time PCR was performed using the LightCycler (Roche Diagnostics) and the QuantiTech SYBR green PCR kit (Qiagen) (11). Sense and antisense primers used were as follows: CATACACCACCACACCTGAAAG and CCGTTTCCTAGTTCTTCCTTGC for CHOP, ATGGCCCGCGCACGCCAGG and CCGCCGCTCGTACTGCGCGTT for Puma, and CAACACAAACCCAAGTCCT and CATTTGCAAACACCCTCCTT for Bim. The data were analyzed using LightCycler software, version 4.0, with all samples normalized to β-actin.
Primary Motoneuron Cultures.
Primary motoneuron cultures were prepared from embryonic day-13 mouse embryos as described in ref. 15. At 10 days in vitro cultures were exposed to 50 μM AMPA (Tocris Cookson) or 3 μM tunicamycin (Bachem). Mitochondrial redox status in cultures was determined using a MTT assay. MTT assay data were supported with direct counts of motoneuron survival in treated cultures assessed using the trypan blue (Sigma) exclusion method. Trypan blue is a blue dye that is excluded from living cells. trypan blue-stained cells are clearly identifiable using a light microscope. After incubation with trypan blue, cultures were immunostained with antibodies to SMI-32 (Abcam), an antibody to nonphosphorylated neurofilaments that labels motoneurons in dissociated spinal cultures. In trypan blue/SMI 32-costained cultures, the number of viable motoneurons was determined by counting only SMI-32-stained motoneurons containing no trypan blue. Values were expressed as a percentage of untreated sister cultures. Caspase-9 activation in puma−/− and WT motoneurons after treatment with AMPA or tunicamycin for 48 h was assessed by immunostaining with antibodies to active caspase 9 (1:300; Calbiochem). The number of caspase 9-positive motoneurons was counted in an area of 0.9 cm2 along a diagonal in 24-well plates. Data are given as mean ± SEM. Statistical significance was assessed using a Mann–Whitney U test, P ≤ 0.05.
Cross-Breeding.
Transgenic SOD1 mice (TgN[SOD1-G93A]1Gur) (3) generated on a C57BL/6 (The Jackson Laboratory) and puma−/− mice (C57BL/6 background) (19) were identified by genotyping from tail DNA (3, 19). To minimize variations in genetic background, two SOD1G93A males were mated with four female puma−/− to generate F1. Four F1 males (puma+/−;SOD1G93A) were then mated with eight puma−/− females to generate all of the genotypes for comparison (46). A total of seven litters were used to generate WT, puma−/−, SOD1G93A, and puma−/−;SOD1G93A mice for this study.
Assessment of Disease Progression.
The body weight, PaGE test, and stride length assessments were carried out blind, twice weekly in 15 mice from each genotype. The PaGE test was repeated three times for each animal (21). Stride length was measured manually and was defined as the distance between successive right-to-right and left-to-left footprints (3). Disease end stage was determined by the inability of the mouse to right itself when placed on its side and by a 20% reduction in body weight compared with body weight at 77 days. Spinal cord sections were stained with gallocyanin, a Nissl stain (15). Gallocyanin-stained sciatic motoneurons >50 μm in diameter and with a clear nucleolus were counted in every third section between L2 and L5 (n = 20 sections). Alternate sections of lumbar spinal cord were immunostained with antibodies to ChAT (22). The number of ChAT-positive motoneurons >50 μm in diameter were counted in every third section between L2 and L5 (n = 20 sections). The counts of ChAT-positive motoneurons were compared with the counts of gallocyanin-stained motoneurons in the same animal. No significant differences were detected in animals examined. Data are given as mean ± SEM. Statistical significance was assessed using a Mann–Whitney U test, P ≤ 0.05.
ACKNOWLEDGMENTS.
This work was supported by Science Foundation Ireland Research Professorship Grant 03/RP1/B344 (to J.H.M.P.); an Irish Research Council for Science, Engineering and Technology Postdoctoral Fellowship (to D.K.); Austrian Science Fund (Fonds zur Förderung der Wissenschaftlichen Forschung) Grants R15 and START (to A.V.); National Health and Medical Research Council (Australia) Program Grant 257502 (to A.S.); Leukemia and Lymphoma Society (New York) SCOR Grant 7015 (to A.S.); and National Cancer Institute/National Institutes of Health Grants CA 80188 and CA 43540 (to A.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707906105/DC1.
Change History
August 31, 2021: Figure 3 has been updated; please see accompanying Correction for details.
References
- 1.Cleveland DW, Rothstein JD. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 2.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 3.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 4.Shibata N, Hirano A, Kobayashi M, Siddique T, Deng HX, Hung WY, Kato T, Asayama K. J Neuropathol Exp Neurol. 1996;55:481–490. doi: 10.1097/00005072-199604000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, et al. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 6.Tobisawa S, Hozumi Y, Arawaka S, Koyama S, Wada M, Nagai M, Aoki M, Itoyama Y, Goto K, Kato T. Biochem Biophys Res Commun. 2003;303:496–503. doi: 10.1016/s0006-291x(03)00353-x. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi H, Almer G, Yamashita S, Guegan C, Nagai M, Xu Z, Sosunov AA, McKhann GM, 2nd, Przedborski S. Proc Natl Acad Sci USA. 2006;103:6025–6030. doi: 10.1073/pnas.0509227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe M, Dykes-Hoberg M, Culotta VC, Price DL, Wong PC, Rothstein JD. Neurobiol Dis. 2001;8:933–941. doi: 10.1006/nbdi.2001.0443. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Sala D, Mollinedo F. J Cell Physiol. 1995;163:523–531. doi: 10.1002/jcp.1041630312. [DOI] [PubMed] [Google Scholar]
- 10.Reimertz C, Kogel D, Rami A, Chittenden T, Prehn JH. J Cell Biol. 2003;162:587–597. doi: 10.1083/jcb.200305149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Concannon CG, Koehler BF, Reimertz C, Murphy BM, Bonner C, Thurow N, Ward MW, Villunger A, Strasser A, Kogel D, et al. Oncogene. 2007;26:1681–1692. doi: 10.1038/sj.onc.1209974. [DOI] [PubMed] [Google Scholar]
- 12.Huang DC, Strasser A. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 13.Ward MW, Kogel D, Prehn JH. J Bionenerg Biomembr. 2004;36:295–298. doi: 10.1023/B:JOBB.0000041756.23918.11. [DOI] [PubMed] [Google Scholar]
- 14.Sharp PS, Dick JR, Greensmith L. Neuroscience. 2005;130:897–910. doi: 10.1016/j.neuroscience.2004.09.069. [DOI] [PubMed] [Google Scholar]
- 15.Kieran D, Hafezparast M, Bohnert S, Dick JR, Martin J, Schiavo G, Fisher EM, Greensmith L. J Cell Biol. 2005;169:561–567. doi: 10.1083/jcb.200501085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oyadomari S, Mori M. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 17.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, et al. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 19.Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Lee B, Lee AS. J Biol Chem. 2006;281:7260–7270. doi: 10.1074/jbc.M509868200. [DOI] [PubMed] [Google Scholar]
- 21.Weydt P, Hong SY, Kliot M, Moller T. NeuroReport. 2003;14:1051–1054. doi: 10.1097/01.wnr.0000073685.00308.89. [DOI] [PubMed] [Google Scholar]
- 22.Oda Y. Pathol Int. 1999;49:921–937. doi: 10.1046/j.1440-1827.1999.00977.x. [DOI] [PubMed] [Google Scholar]
- 23.Rothstein JD, Tsai G, Kuncl RW, Clawson L, Cornblath DR, Drachman DB, Pestronk A, Stauch BL, Coyle JT. Ann Neurol. 1990;28:18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- 24.Trotti D, Rolfs A, Danbolt NC, Brown RH, Jr, Heidger MA. Nat Neurosci. 1999;2:427–433. doi: 10.1038/8091. [DOI] [PubMed] [Google Scholar]
- 25.Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, Rapoport TA, Ploegh HL. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 26.Plemper RK, Bohmler S, Bordallo J, Sommer T, Wolf DH. Nature. 1997;388:891–895. doi: 10.1038/42276. [DOI] [PubMed] [Google Scholar]
- 27.Kabashi E, Agar JN, Taylor DM, Minotti S, Durham HD. J Neurochem. 2004;89:1325–1335. doi: 10.1111/j.1471-4159.2004.02453.x. [DOI] [PubMed] [Google Scholar]
- 28.Marciniak J, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vlug AS, Teuling E, Haasdijk ED, French P, Hoogenraad CC, Jaarsma D. Eur J Neurosci. 2005;22:1881–1894. doi: 10.1111/j.1460-9568.2005.04389.x. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi H, Wang HG. J Biol Chem. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 31.Nakano K, Vousden KH. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 32.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. Mol Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 33.Han J, Flemington C, Houghton AB, Gu Z, Zambetti GP, Lutz RJ, Zhu L, Chittenden T. Proc Natl Acad Sci USA. 2001;98:11318–11323. doi: 10.1073/pnas.201208798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuntz C, Kinoshita Y, Flint Beal M, Donehower LA, Morrison RS. Exp Neurol. 2000;165:184–190. doi: 10.1006/exnr.2000.7464. [DOI] [PubMed] [Google Scholar]
- 35.Prudlo J, Koenig J, Graser J, Burckhardt E, Mestres P, Menger M, Roemer K. Brain Res. 2000;879:183–187. doi: 10.1016/s0006-8993(00)02745-1. [DOI] [PubMed] [Google Scholar]
- 36.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, et al. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 37.Lei K, Davis RJ. Proc Natl Acad Sci USA. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veglianese P, Lo Coco D, Bao Cutrona M, Magnoni R, Pennacchini D, Pozzi B, Gowing G, Julien JP, Tortarolo M, Bendotti C. Mol Cell Neurosci. 2006;31:218–231. doi: 10.1016/j.mcn.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Hetz C, Thielen P, Fisher J, Pasinelli P, Brown RH, Korsmeyer S, Glimcher L. Cell Death Differ. 2007;14:1386–1389. doi: 10.1038/sj.cdd.4402166. [DOI] [PubMed] [Google Scholar]
- 40.Salvesen GS, Dixit VM. Proc Natl Acad Sci USA. 1999;96:10964–10967. doi: 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raoul C, Estevez AG, Nishimune H, Cleveland DW, deLapeyriere O, Henderson CE, Haase G, Pettmann B. Neuron. 2002;35:1067–1083. doi: 10.1016/s0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- 42.Guegan C, Vila M, Teismann P, Chen C, Onteniente B, Li M, Friedlander RM, Przedborski S. Mol Cell Neurosci. 2002;20:553–562. doi: 10.1006/mcne.2002.1136. [DOI] [PubMed] [Google Scholar]
- 43.Gould TW, Buss RR, Vinsant S, Prevette D, Sun W, Knudson CM, Milligan CE, Oppenheim RW. J Neurosci. 2006;26:8774–8786. doi: 10.1523/JNEUROSCI.2315-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vila M, Przedborski S. Nat Rev Neurosci. 2003;4:365–375. doi: 10.1038/nrn1100. [DOI] [PubMed] [Google Scholar]
- 45.Brockington A, et al. J Neuropath Exp Neurol. 2006;65:26–36. doi: 10.1097/01.jnen.0000196134.51217.74. [DOI] [PubMed] [Google Scholar]
- 46.Kang SJ, Sanchez I, Jing N, Yuan J. J Neurosci. 2003;23:5455–5460. doi: 10.1523/JNEUROSCI.23-13-05455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]