Abstract
Hydrogen sulfide (H2S) is naturally produced in animal cells. Exogenous H2S has been shown to effect physiological changes that improve the capacity of mammals to survive in otherwise lethal conditions. However, the mechanisms required for such alterations are unknown. We investigated the physiological response of Caenorhabditis elegans to H2S to elucidate the molecular mechanisms of H2S action. Here we show that nematodes exposed to H2S are apparently healthy and do not exhibit phenotypes consistent with metabolic inhibition. Instead, animals exposed to H2S are thermotolerant and long-lived. These phenotypes require SIR-2.1 activity but are genetically independent of the insulin signaling pathway, mitochondrial dysfunction, and caloric restriction. These studies suggest that SIR-2.1 activity may translate environmental change into physiological alterations that improve survival. It is interesting to consider the possibility that the mechanisms by which H2S increases thermotolerance and lifespan in nematodes are conserved and that studies using C. elegans may help explain the beneficial effects observed in mammals exposed to H2S.
Early life arose in highly reducing conditions where hydrogen sulfide (H2S) was abundant and molecular oxygen (O2) was scarce (1, 2). Early organisms were likely to have extracted energy from the environment initially by anoxygenic photosynthesis using chemicals, including sulfide, as electron donors, followed by anaerobic respiration using sulfate as an electron acceptor (resulting in the production of sulfide), and, finally, by aerobic respiration (1). The success of primordial eukaryotes may have resulted from their ability to take advantage of chemically unstable mixtures of H2S and O2. In modern marine sediments, eukaryotic microbes accumulate at locations where O2 and H2S coexist, allowing for maximal energy production by redox chemistry (1, 3).
The importance of O2 in biology is widely recognized; however, the ancient nature of H2S suggests that it also might impact fundamental aspects of biological processes. H2S is naturally produced by animal cells, and endogenous H2S affects various aspects of physiology (4, 5). In addition, recent evidence suggests that H2S can have dramatic effects on mammalian physiology (6–8). Mice exposed to H2S enter into a physiological state that allows them to endure periods of low metabolic rate and decreased core body temperature without apparent ill effects (6). The H2S-induced state also allows for mice to survive exposure to otherwise lethal hypoxic conditions (7). In rats, H2S improves outcome after severe hemorrhage (50). These studies raise the possibility that exogenous H2S could be of clinical benefit, especially for pathologies resulting from decreased oxygen perfusion. It is unlikely that there is a single cellular target of H2S that mediates all observed physiological alterations. H2S is a strong reducing agent that could reduce disulfide bonds of proteins (or maintain thiol groups in the reduced state), interact with active-site cysteine residues, affect cellular redox balance, or bind to metal atoms in enzymes (4, 9). In addition, it was recently demonstrated that H2S can act as an electron donor and increase production of ATP in metazoans (10, 11), as was shown previously for the gutless clam Solemya reidi (12, 13). These examples illustrate that H2S will probably have many cellular targets, just as O2 interacts with myriad cellular factors to influence diverse cellular processes.
To investigate the nature of H2S-induced physiological alterations in animals, we exposed the genetically tractable nematode Caenorhabditis elegans to atmospheres containing H2S. As expected, we found that high concentrations of H2S are toxic. However, exposure to low concentrations of H2S results in physiological alterations that allow for increased thermotolerance and lifespan. We show that these phenotypes require the NAD+-dependent deacetylase sir-2.1, suggesting that one cellular activity of H2S is to increase the activity of SIR-2.1.
Results and Discussion
C. elegans is not adversely affected when grown in atmospheres containing 50-ppm H2S (0.005%) in room air (hereafter referred to simply as H2S) [supporting information (SI) Fig. 5]. We chose 50-ppm H2S because this concentration has been shown to affect mammalian physiology (6) but is not apparently toxic to the worms. Animals raised in H2S are visually indistinguishable from untreated controls and produce statistically identical numbers of progeny (221 ± 35 in H2S, compared with 234 ± 15 in control conditions; P > 0.05; n = 5–10 in each group). Neither embryonic nor postembryonic development is delayed by H2S (Table 1). In addition, the rate of egg-laying is not significantly different in H2S (Table 2). The rate of egg-laying is tightly correlated with oocyte production (14), an energetically expensive activity that is a sensitive readout of metabolic capacity. Consistent with this, we observe a 2-fold decrease in the rate of egg-laying when ambient O2 tension is reduced to 2% (from 21% O2 in room air), a perturbation that was previously shown to decrease the metabolic rate of worms by ≈30% (15). H2S does not further alter the rate of egg-laying in environments with reduced ambient O2 (Table 2). These data contrast with hypometabolic phenotypes commonly observed in nematodes with defective, including clk-1(qm30) mitochondrial function, including clk-1(qm30) animals (16–19). Our experiments demonstrate that apparent metabolic output is not appreciably changed when animals are raised in H2S. However, we cannot definitively conclude that mitochondrial energy production has not been affected in these conditions. In addition, we observe that H2S exposure does not induce expression of several transgenes driven by heat-shock promoters, including hsp-16.2::GFP and hsp-4::GFP (SI Fig. 6) (20–23). Together these data indicate that animals grown in H2S are as healthy as untreated controls, and that in our conditions this concentration of H2S does not affect apparent metabolic rate.
Table 1.
Developmental rate in H2S
| Developmental time, h ± SEM (n) |
||
|---|---|---|
| Embryogenesis | Postembryonic | |
| Untreated | 12.6 ± 0.4 (43) | 49.6 ± 0.2 (15) |
| H2S | 13.3 ± 0.4 (34) | 49.3 ± 0.2 (15) |
| clk-1(qm30) | 16.9 ± 2.0 (17)* | 69.0 ± 0.3 (15)* |
*Developmental time is significantly different (P < 0.05) from untreated controls by log-rank analysis.
Table 2.
Rate of egg-laying in H2S
| Rate of egg-laying, no. of embryos per h ± SD |
||||
|---|---|---|---|---|
| Room air | H2S | 2% O2 | 2% O2 plus H2S | |
| Untreated | 7.8 ± 1.5 | 8.3 ± 3.0 | 4.2 ± 1.3 | 4.5 ± 1.6 |
| H2S | nd | 7.8 ± 2.0 | 4.9 ± 1.4 | 4.7 ± 1.2 |
| clk-1(qm30) | 4.2 ± 0.6* | 3.2 ± 1.1* | 2.4 ± 0.8* | 2.5 ± 1.0* |
nd, not done.
*Rate of egg-laying is significantly different (P < 0.05) than untreated controls in same conditions by Student's t test.
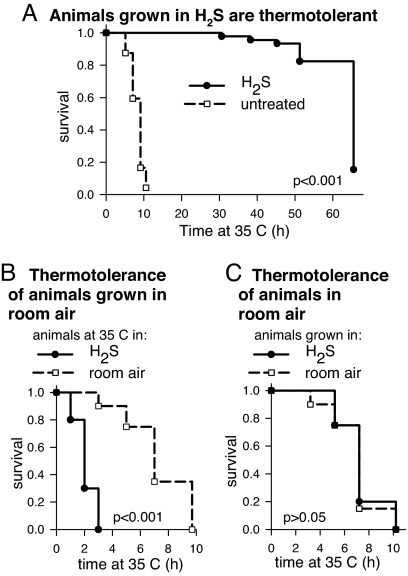
We observed that animals grown in H2S are more thermotolerant than untreated controls (Fig. 1). At high temperature, animals grown in H2S have a mean survival time up to 8-fold longer than untreated controls. Although the maximum extension of survival time observed varied between experiments, the effect was quite robust, with an average of 77% of H2S-treated animals alive when all untreated animals had died (15 independent experiments) (SI Fig. 7). In this experiment, animals were raised in H2S and challenged with high temperature in the presence of H2S. In fact, we observe that animals grown in room air were more sensitive to thermal stress in H2S (Fig. 1B). Thus, H2S does not act directly to prevent damage associated with thermal stress. Moreover, unlike thermotolerance induced by prior stress such as heat shock (24) or azide (25), continuous exposure to H2S is required for increased thermotolerance (Fig. 1C). These data suggest that H2S exposure initiates physiological alterations, one manifestation of which is increased survival at high temperature.
Fig. 1.
H2S increases thermotolerance in wild-type C. elegans. (A) Animals exposed to H2S survive longer than untreated controls at high temperature. Nematodes were moved to 35°C in the same gaseous atmosphere in which they had been cultured (SI Fig. 5). The mean survival time of animals grown in H2S was 65.5 h (solid line; n = 136), compared with 9.1 h (n = 96) for untreated controls (dashed line). (B) Prior exposure to H2S is required to survive high temperature in H2S. All animals were grown in room air without H2S and then moved to 35°C in the presence or absence of 50-ppm H2S. Animals first exposed to H2S at high temperature had a mean survival time of 2.1 h (solid line; n = 20), whereas the control group exposed in room air survived for 7.3 h (dashed line; n = 20). (C) The continuous presence of H2S in the atmosphere is required for increased survival at high temperature. Animals were exposed to 35°C in room air. Animals grown in H2S before heat shock survived 7.3 h (solid line; n = 20), which is not significantly longer than untreated controls (dashed line; 7.0 h; n = 20). Indicated P values were determined by log-rank analysis.
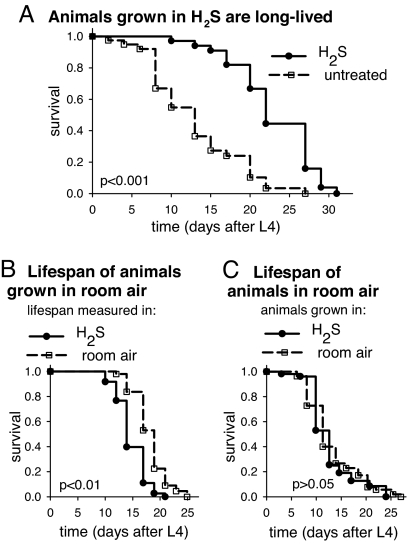
In C. elegans, resistance to high temperature is often correlated with increased lifespan (24). Indeed, we observe that animals grown in H2S are long-lived compared with controls (Fig. 2). The mean lifespan of animals grown in H2S is 9.6 days greater than the untreated population, an increase of 70%. Maximum lifespan was similarly increased as H2S-treated animals reached 75% mortality 10 days after the control population. Increased lifespan is not observed when animals are moved into H2S at the beginning of the lifespan experiment as L4 larvae. In fact, the lifespan of these animals is slightly shorter than untreated controls (Fig. 2B). These data suggest that H2S cannot act postdevelopmentally to slow the rate of aging (see also SI Fig. 7). However, these animals produce normal numbers of progeny, suggesting that overall physiological function is not impaired (227 ± 18 progeny when moved to H2S, compared with 208 ± 16 in room air; P > 0.05, n = 5 in each group). Lifespan extension requires the continuous presence of H2S in the atmosphere insofar as animals grown in H2S but moved to room air have a normal lifespan (Fig. 2C). This finding indicates that H2S exposure solely during development is insufficient for increased lifespan. We conclude that the increase in lifespan is another manifestation of the physiological alterations resulting from the exposure to H2S.
Fig. 2.
H2S increases lifespan in C. elegans. (A) Animals grown in H2S live longer than untreated controls. The lifespan of animals was monitored in the same conditions in which they had developed. The mean lifespan of animals in H2S was 22.6 ± 1.0 days (solid line; n = 80), compared with 13.0 ± 1.0 days for untreated controls in room air (dashed line; n = 40). Maximum lifespan also was increased. (B) Exposure to H2S beginning as L4 does not increase lifespan. All animals were from populations grown in room air. The lifespan of animals moved into H2S-containing environments at the beginning of the lifespan experiment (solid line) is 14.8 ± 0.3 days (n = 73), which is slightly shorter than controls that remained in house air (dashed line; mean lifespan 18.2 ± 0.4 days; n = 48). (C) Increased lifespan requires continuous exposure to H2S. The lifespan of all animals was monitored in room air. The lifespan of animals raised in H2S until L4 (solid line; 12.8 ± 0.7 days; n = 52) was indistinguishable from untreated controls (dashed line; 13.2 ± 0.7 days; n = 59). All lifespan experiments were performed at room temperature.
Most genes that influence lifespan can be categorized into one of three genetically independent pathways in C. elegans (26–28). We considered the possibility that exposure to H2S and associated physiological alterations may modulate one or more of these pathways. To evaluate this possibility, we tested whether exposure to H2S caused increased thermotolerance in mutant animals with defects in these pathways.
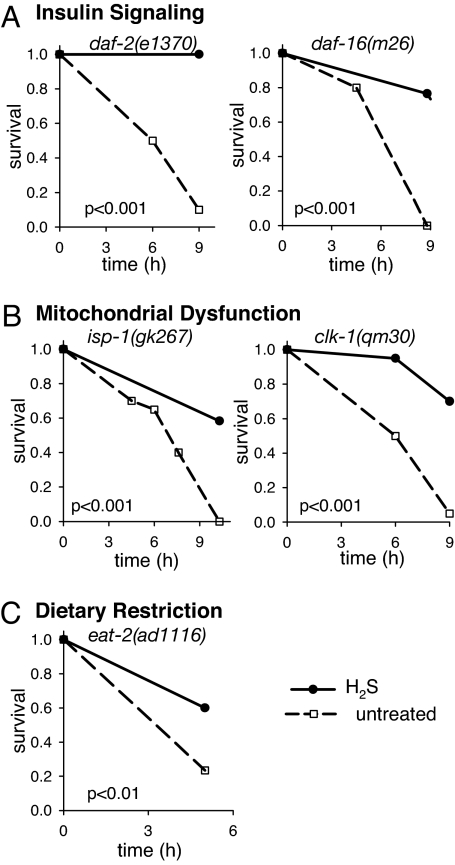
In C. elegans, the insulin/IGF signaling (IIS) pathway regulates the decision to enter into an alternative third larval stage, the dauer, upon exposure to unfavorable conditions, such as high population density, low food, or high temperature (29). Mutations in the insulin-like receptor DAF-2 that reduce IIS increase the probability of entry into the dauer state and, in adults, increase thermotolerance and lengthen lifespan even without entry into dauer (30–32). All known phenotypes of daf-2 mutants can be suppressed by mutations in the DAF-16 FOXO transcription factor (26, 30, 31). Our data suggest that exposure to H2S does not result in decreased IIS. First, H2S exposure starting in adulthood does not increase lifespan (Fig. 2B), whereas knockdown of daf-2 by RNAi starting in adulthood is sufficient to increase lifespan (32). Second, daf-2-mutant animals are more resistant to high temperature when grown in H2S (Fig. 3A). Third, H2S does not induce entry into the dauer state in wild-type nematodes because postembryonic development time is not extended (Table 1), nor does it affect entry into or exit from dauer in daf-2(e1370) mutants (data not shown). Finally, daf-16-mutant animals become thermotolerant upon exposure to H2S (Fig. 3A). These data suggest that the mechanism by which H2S increases lifespan and thermotolerance is independent of the IIS pathway.
Fig. 3.
Thermotolerance of canonical long-lived mutants is increased by H2S. Just as H2S increases thermotolerance of wild-type worms (Fig. 1 and SI Fig. 6), long-lived mutants in canonical pathways that influence lifespan (20) also are more thermotolerant when grown in H2S. All animals grown in H2S were challenged with high temperature in H2S (solid line), whereas the thermotolerance of untreated controls was assayed in room air (dashed line). (A) H2S effects are genetically independent of IIS. The thermotolerance of daf-2(e1370) animals can be enhanced by exposure to H2S, and daf-16(m26) mutants, which are defective in IIS, become thermotolerant when grown in H2S. To facilitate the experiments shown in this figure, strains that show intrinsic thermotolerance, such as daf-2(e1370) (30), were tested at a slightly higher temperature both in room air and H2S. However, the thermotolerance at 35°C also is increased when the animals are grown in H2S (data not shown). (B) H2S-induced thermotolerance is observed in isp-1(gk267) and clk-1(qm30) animals that are long-lived as a result of mitochondrial dysfunction. (C) H2S-induced thermotolerance is observed in eat-2(ad1116) mutant animals, which have defects in pharyngeal pumping that result in dietary restriction.
Reduction of mitochondrial function is a well established mechanism for increasing the lifespan of C. elegans (27). In vitro, H2S is an inhibitor of cytochrome c oxidase, the terminal enzyme in the electron transport chain (9). However, we do not observe hypometabolic phenotypes in animals grown in H2S (Tables 1 and 2), suggesting that mitochondrial function is not grossly affected in these conditions. In addition, depletion of mitochondrial components by RNAi only during development increases lifespan (16), whereas animals grown in H2S, but moved to room air as adults, are not long-lived (Fig. 2C). These data suggest that H2S exposure has characteristics distinct from mitochondrial dysfunction. In support of this premise, we observe that isp-1- and clk-1-mutant animals, which have defects in mitochondrial function and are long-lived (17, 18), become more resistant to high temperature when grown in H2S (Fig. 3B). We conclude from these data that the effect of H2S on lifespan is mediated by a genetic mechanism distinct from mitochondrial dysfunction.
Reduced caloric intake or dietary restriction (DR) extends lifespan in a wide range of organisms (33). C. elegans subjected to DR appear thin and pale, develop slowly, and have reduced fecundity, which are phenotypes not observed in animals grown in H2S (Tables 1 and 2) (19, 34). Furthermore, DR can increase lifespan when initiated in adults (35–37), whereas H2S exposure cannot (Fig. 2B). Therefore, we consider it unlikely that H2S acts through the DR pathway. Consistent with this interpretation, eat-2-mutant animals, which are long-lived because of DR (38), become thermotolerant upon exposure to H2S (Fig. 3C). We conclude that H2S alters the physiology of worms in a manner distinct from DR, suggesting that it acts through a separate mechanism.
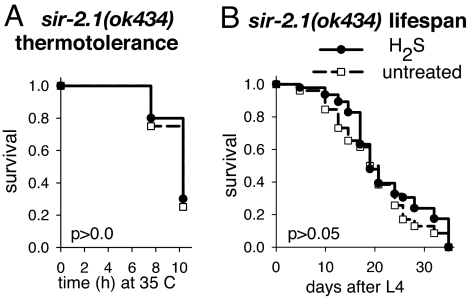
In addition to, but perhaps overlapping with, these genetically defined pathways, Sir2 homologues influence lifespan in many organisms, including C. elegans (39–42). Overexpression of the C. elegans Sir2 homologue, sir-2.1, increases lifespan by 18–50% (43). Our data indicate that sir-2.1 is required for increased thermotolerance and lifespan upon exposure to H2S. In contrast to wild type (Figs. 1A and 2A), the thermotolerance and lifespan of nematodes harboring a deletion in the sir-2.1 gene are unchanged when the animals are grown in H2S (Fig. 4). However, we consider it unlikely that H2S results in increased lifespan as a result of increased SIR-2.1 expression. H2S effects on lifespan are independent of daf-16 (Fig. 3A), whereas lifespan extension by overexpression of sir-2.1 requires DAF-16 activity (43). Indeed, sir-2.1 transcript levels in animals grown in H2S are indistinguishable from untreated controls as measured by quantitative RT-PCR, and animals overexpressing sir-2.1 become more thermotolerant when grown in H2S (SI Fig. 8). We conclude that H2S modulates SIR-2.1 activity to impart increased thermotolerance and lifespan in a manner distinct from sir-2.1 overexpression. The fact that these phenotypes require sir-2.1 supports the interpretation that the effects of H2S are distinct from DR because increased lifespan resulting from DR does not require sir-2.1 (44). Moreover, the finding that SIR-2.1 activity is required for increased thermotolerance and lifespan in H2S further suggests that these phenotypes do not result from nonspecific metabolic suppression.
Fig. 4.
sir-2.1 is required for increased thermotolerance and lifespan in H2S. (A) H2S does not increase thermotolerance of animals that have a deletion in sir-2.1. The mean survival time of sir-2.1(ok434) animals grown in H2S and exposed to high temperature in H2S (solid line) is 9.8 ± 0.3 h (n = 20), which is not significantly longer than untreated controls in room air (dashed line; mean survival 9.6 ± 0.3 h; n = 20). (B) H2S does not increase the lifespan of sir-2.1(ok434) animals. The lifespan of sir-2.1(ok434) animals raised in H2S is 20.0 ± 1.6 days (solid line; n = 47), which is statistically indistinguishable from control animals in room air (dashed line; 22.2 ± 1.2 days; n = 26). Indicated P values were determined by log-rank analysis.
Sir2 homologues are NAD+-dependent deacetylase enzymes that may have a variety of substrates (45). This finding raises the possibility that H2S shifts redox homeostasis, thereby increasing the available NAD+ (or the NAD+/NADH ratio) and resulting in increased SIR-2.1 activity (40, 46). Alternatively, H2S may directly modify SIR-2.1 to alter its activity (47). It also is possible that SIR-2.1 is indirectly activated by some other aspect of H2S-induced physiological alterations. Whatever the mechanism by which H2S-induced physiological alterations are translated into the phenotype of increased lifespan, our studies raise the possibility that endogenous H2S naturally regulates SIR-2.1 activity. It may be that Sir2 homologues are involved in mediating the physiological alterations observed in mammals exposed to exogenous H2S. Further investigations of genetic mechanisms that mediate H2S-induced phenotypes in nematodes may yield insights into similar mechanisms in higher organisms, including humans, with potentially wide-ranging implications in both basic research and clinical practice.
Materials and Methods
Growing Nematodes in H2S-Containing Atmospheres.
Bristol strain N2 (wild-type) and mutant nematode strains were grown at room temperature on nematode growth medium (NGM) plates seeded with live Escherichia coli OP50 food (48). Mutant strains obtained from the C. elegans genetic stock center were as follows: CB130, daf-2(e1370); DR26, daf-16(m26); VC520, isp-1(gk267); MQ130, clk-1(qm30); DA1116, eat-2(ad1116); and VC199, sir-2.1(ok434).
Plates were maintained in atmospheric chambers sealed with Dow Corning Vacuum Grease (Sigma–Aldrich). Care was taken to ensure that cultures did not starve. Chambers were continuously perfused with room air or 50-ppm H2S that was freshly mixed into room air (SI Fig. 5). Gasses were hydrated by using gas wash bottles (Fisher) and moved through one-eighth-inch outer diameter FEP tubing (Cole Parmer) with connections by snap connectors (Cole Parmer), stainless-steel quick-connect fittings, or compression fittings (Seattle Fluid Systems). The H2S-containing atmospheres were constructed by mixing 5,000-ppm H2S (balanced with N2) with room air by using mass flow controllers (model no. 810 and Smart-Trak Series 100; Sierra Instruments). All compressed gas mixtures used in this study were obtained from Byrne Gas and were certified standard to within 2% of indicated concentration. Flow tubes (Aalborg) were used to distribute the gas mixture to different chambers. Gas flow rate was 100 cm3/m to small boxes (100–300 ml) and 800 cm3/m to the large boxes (1–3 liters) used to culture nematode strains at room temperature. At these flow rates, the gaseous environment of the atmospheric chambers is exchanged every 20–30 min. The concentration of H2S was monitored with a custom-built H2S detector (J. Rivera, Fred Hutchinson Cancer Research Center) containing a three-electrode electrochemical Surecell H2S detector (Sixth Sense). The detector was zeroed with room air and spanned with 100-ppm H2S before each use. Data were collected by using Chart software with a Powerlab data acquisition unit (ADInstruments) and analyzed with EXCEL. The concentration of H2S measured was consistently within 10 ppm of the reported value and was stable from day to day. The H2S-containing atmospheres did not alter the pH of the NGM plates.
Brood Size Measurement.
To determine the number of viable progeny produced by nematodes, individual fourth-stage larvae (L4) were transferred to NGM plates with OP50 food at room temperature. Animals were moved daily until they quit laying fertilized eggs. Progeny were counted as L4/young adult.
Measuring Developmental Rates.
The time required for embryonic development was determined by measuring the time required for two-cell embryos to hatch. Two-cell embryos were isolated from log-phase adults as previously described (49). Briefly, adults were chopped with a razor blade and ≈20 two-cell embryos were moved to NGM plates without food by mouth pipette. The number of embryos that hatched was monitored every 45–60 min beginning 6–8 h after embryos were picked. Embryos that did not hatch after 36 h were considered dead and were not included in the analysis. Median time of development was determined by log-rank analysis in SigmaStat (Systat). Data from one representative experiment for both embryonic and postembryonic development are shown in Table 1, although each experiment was repeated several times with similar results.
Postembryonic development was measured as the time required for starved first-stage larvae (L1) to become gravid, egg-laying adults. Starved L1 were isolated by picking 30–50 adults from each population into 10 μl of hypochlorite solution (2.5 N KOH, 5% NaOCl) on a small (unseeded) NGM plate. After 5 min, 1 ml of M9 buffer (48) was added to the plate, and the embryos were returned to the atmospheric chambers. After 24–36 h, starved L1 were moved onto NGM plates with OP50, returned to the chamber, and allowed to develop at room temperature. After 30–48 h, individual larvae were moved to NGM plates with a 10-μl spot of OP50. Each worm was monitored every 6–12 h until it began laying eggs (intervals became closer as time progressed and other animals became gravid). If more than one embryo had been laid, the time that the first egg was laid was determined assuming that one egg was laid every 15 min for wild type and every 30 min for the clk-1(qm30) mutants. This value was determined empirically by counting the number of embryos laid by each worm for the 6-h period after it began egg-laying. Data were analyzed by using log-rank analysis in SigmaStat (Systat).
The rate of egg-laying was determined for first-day gravid adults from populations cultured in each condition (room air with or without 50-ppm H2S). Animals were picked as L4 from mixed-stage populations and allowed to develop for 20–30 h in the same conditions at room temperature. Individual worms were then placed onto NGM plates with a 10-μl spot of OP50 food. The number of embryos laid in 3–5 h was counted to determine the rate of egg-laying. To create an atmosphere with 2% O2, N2 was mixed with 5% O2 balanced with N2. Smart-Trak series 100 mass flow controllers (Sierra Instruments) were used to mix the gas and split it into two atmospheric chambers. Using a model 810 mass flow controller (Sierra Instruments), we then added H2S to the 2% O2 that flowed into one of the chambers. Student's t test was used to determine whether the rate of egg-laying varied significantly between conditions, assuming two-tailed distributions with unequal variance (EXCEL). In each experiment, 10–15 individuals were included in each group. The data shown in Table 2 are from one experiment that is representative of at least three independent assays.
Thermotolerance Assay.
Cultures of nematodes were established in 50-ppm H2S or room air control conditions and maintained for at least 1 week before thermotolerance measurement. Care was taken to prevent the population from starving. Nematodes were picked from these mixed-stage populations as L4 larvae and allowed to develop for 24–48 h at room temperature. However, treated and control animals were always the same age. For temperature-sensitive daf-2(e1370) mutants (30), cultures were maintained in H2S-containing environments at 17°C and moved to room temperature as L4. Groups of 20–30 animals were transferred to NGM plates without food and then placed into an atmospheric chamber perfused with the indicated gas at high temperature in an Echotherm IN35 incubator [National Institute of Standards and Technology (NIST)-traceable; Torrey Pines Scientific] or a VWR incubator model 2005 (VWR International). A ring of palmitic acid around the edge of the plate helped prevent the worms escaping the surface of the agar. Temperature was maintained at 34.5 ± 1°C, although the temperature was raised slightly to facilitate experiments with thermotolerant strains. In these experiments, the high temperature was chosen so that controls died in <10 h [in Fig. 3A, daf-2(e1370) animals were tested at 36.5°C]. HOBO U10 data loggers that were calibrated to a NIST-traceable thermometer (Onset Corporation) were used to monitor the temperature in each chamber. In every experiment, the temperature of the room air- and H2S-containing chambers was the same. Plates were removed to count the number of animals that had died every few hours. Nematodes were considered dead and removed from the plate when they no longer responded to prodding with a platinum wire. Kaplan–Meier log-rank tests with the program SigmaStat (Systat) were used to evaluate statistical significance. Animals that could not be accounted for were censored from the analysis. Each assay was repeated at least twice with similar results.
Lifespan Measurements.
On day 0, groups of 20–30 L4 animals from populations in each condition were picked from mixed-stage cultures onto NGM plates seeded with live OP50 and spotted with 25 μl of 50 μg/ml 5-fluoro-2′-deoxyuridine (Sigma–Aldrich) to prevent growth of progeny. Control experiments indicated that the lifespan of nematodes in H2S was not affected by the 5-fluoro-2′-deoxyuridine. Plates were placed into atmospheric chambers at room temperature. Every few days, the plates were removed from the chambers to monitor the number of animals remaining alive. Nematodes were considered dead when they no longer responded to repeated prodding with a platinum wire. Data were analyzed with SigmaStat (Systat) by using Kaplan–Meier survival analysis. Each assay was repeated at least twice with similar results.
Supplementary Material
ACKNOWLEDGMENTS.
We thank members of the laboratories of J. Priess and M.B.R. at the Fred Hutchinson Cancer Research Center for discussion of this project and critical reading of the manuscript and E. Blackstone for assistance with the initial investigations of H2S in nematodes. This work was supported by National Institutes of Health Grant R01 GM48435 (to M.B.R.), National Research Service Award Fellowship 1F32FM073369 (to D.L.M.), the Caenorhabditis Genetics Center, and the National Institutes of Health/National Center for Research Resources.
Footnotes
Conflict of interest statement: The authors acknowledge a potential conflict of interest in that both authors are named as inventors on at least one patent that was licensed to a private company, founded by Mark Roth, to commercialize this technology.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710191104/DC1.
References
- 1.Fenchel T, Finlay BJ. Ecology and Evolution in Anoxic Worlds. Oxford: Oxford Univ Press; 1995. [Google Scholar]
- 2.Krauth-Siegel RL, Jockers-Scherubl MC, Becker K, Schirmer RH. Biochem Soc Trans. 1989;17:315–317. doi: 10.1042/bst0170315. [DOI] [PubMed] [Google Scholar]
- 3.Behnke A, Bunge J, Barger K, Breiner HW, Alla V, Stoeck T. Appl Environ Microbiol. 2006;72:3626–3636. doi: 10.1128/AEM.72.5.3626-3636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowicka E, Beltowski J. Pharmacol Rep. 2007;59:4–24. [PubMed] [Google Scholar]
- 5.Stipanuk MH. Ann Rev Nutrition. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 6.Blackstone E, Morrison M, Roth MB. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 7.Blackstone E, Roth MB. Shock. 2007;27:370–372. doi: 10.1097/SHK.0b013e31802e27a0. [DOI] [PubMed] [Google Scholar]
- 8.Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, Moore PK. J Pharmacol Exp Ther. 2006;316:670–678. doi: 10.1124/jpet.105.092023. [DOI] [PubMed] [Google Scholar]
- 9.Beauchamp RO, Jr, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA. Crit Rev Toxicol. 1984;13:25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- 10.Goubern M, Andriamihaja M, Nubel T, Blachier F, Bouillaud F. FASEB J. 2007;21:1699–1706. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- 11.Yong R, Searcy DG. Comp Biochem Physiol B Biochem Mol Biol. 2001;129:129–137. doi: 10.1016/s1096-4959(01)00309-8. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien J, Vetter RD. J Exp Biol. 1990;149:133–148. doi: 10.1242/jeb.149.1.133. [DOI] [PubMed] [Google Scholar]
- 13.Powell MA, Somero GN. Science. 1986;233:563–566. doi: 10.1126/science.233.4763.563. [DOI] [PubMed] [Google Scholar]
- 14.McCarter J, Bartlett B, Dang T, Schedl T. Dev Biol. 1999;205:111–128. doi: 10.1006/dbio.1998.9109. [DOI] [PubMed] [Google Scholar]
- 15.Van Voorhies W, Ward S. J Exp Biol. 2000;203:2467–2478. doi: 10.1242/jeb.203.16.2467. [DOI] [PubMed] [Google Scholar]
- 16.Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- 17.Feng J, Bussiere F, Hekimi S. Dev Cell. 2001;1:633–644. doi: 10.1016/s1534-5807(01)00071-5. [DOI] [PubMed] [Google Scholar]
- 18.Wong A, Boutis P, Hekimi S. Genetics. 1995;139:1247–1259. doi: 10.1093/genetics/139.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen M, Hsu AL, Dillin A, Kenyon C. PLoS Genet. 2005;1:119–128. doi: 10.1371/journal.pgen.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE. Nat Genet. 2005;37:894–898. doi: 10.1038/ng1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong M, Kwon JY, Shim J, Lee J. J Mol Biol. 2004;344:369–381. doi: 10.1016/j.jmb.2004.09.077. [DOI] [PubMed] [Google Scholar]
- 22.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 23.Shen X, Ellis RE, Lee K, Liu CY, Yang K, Solomon A, Yoshida H, Morimoto R, Kurnit DM, Mori K, Kaufman RJ. Cell. 2001;107:893–903. doi: 10.1016/s0092-8674(01)00612-2. [DOI] [PubMed] [Google Scholar]
- 24.Lithgow G, White T, Melov S, Johnson T. Proc Natl Acad Sci USA. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massie MR, Lapoczka EM, Boggs KD, Stine KE, White GE. Cell Stress Chaperones. 2003;8:1–7. doi: 10.1379/1466-1268(2003)8<1:ettmis>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenyon C. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Wolff S, Dillin A. Exp Gerontol. 2006;41:894–903. doi: 10.1016/j.exger.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 28.Hekimi S, Burgess J, Bussiere F, Meng Y, Benard C. Trends Genet. 2001;17:712–718. doi: 10.1016/s0168-9525(01)02523-9. [DOI] [PubMed] [Google Scholar]
- 29.Golden JW, Riddle DL. Dev Biol. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- 30.Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, Riddle DL. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 32.Dillin A, Crawford DK, Kenyon C. Science. 2002;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- 33.Walker G, Houthoofd K, Vanfleteren JR, Gems D. Mech Ageing Dev. 2005;126:929–937. doi: 10.1016/j.mad.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Shibata Y, Fujii T, Dent JA, Fujisawa H, Takagi S. Genetics. 2000;154:635–646. doi: 10.1093/genetics/154.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klass MR. Mech Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 36.Houthoofd K, Braeckman BP, Johnson TE, Vanfleteren JR. Exp Gerontol. 2003;38:947–954. doi: 10.1016/s0531-5565(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 37.Kaeberlein TL, Smith ED, Tsuchiya M, Welton KL, Thomas JH, Fields S, Kennedy BK, Kaeberlein M. Aging Cell. 2006;5:487–494. doi: 10.1111/j.1474-9726.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- 38.Lakowski B, Hekimi S. Proc Nat Acad Sci USA. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bordone L, Guarente L. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 40.Longo VD, Kennedy BK. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Guarente L. Mech Ageing Dev. 2005;126:923–928. doi: 10.1016/j.mad.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 42.Guarente L, Picard F. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 43.Tissenbaum HA, Guarente L. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 44.Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- 45.Haigis MC, Guarente LP. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 46.Blander G, Guarente L. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 47.Ziegler DM. Annu Rev Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]
- 48.Brenner S. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nystul TG, Roth MB. Proc Nat Acad Sci USA. 2004;101:9133–9136. doi: 10.1073/pnas.0403312101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morrison M, Blackwood JE, Lockett SL, Iwata A, Winn RK, Roth MB. J Trauma. doi: 10.1097/TA.0b013e3181507579. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.