Abstract
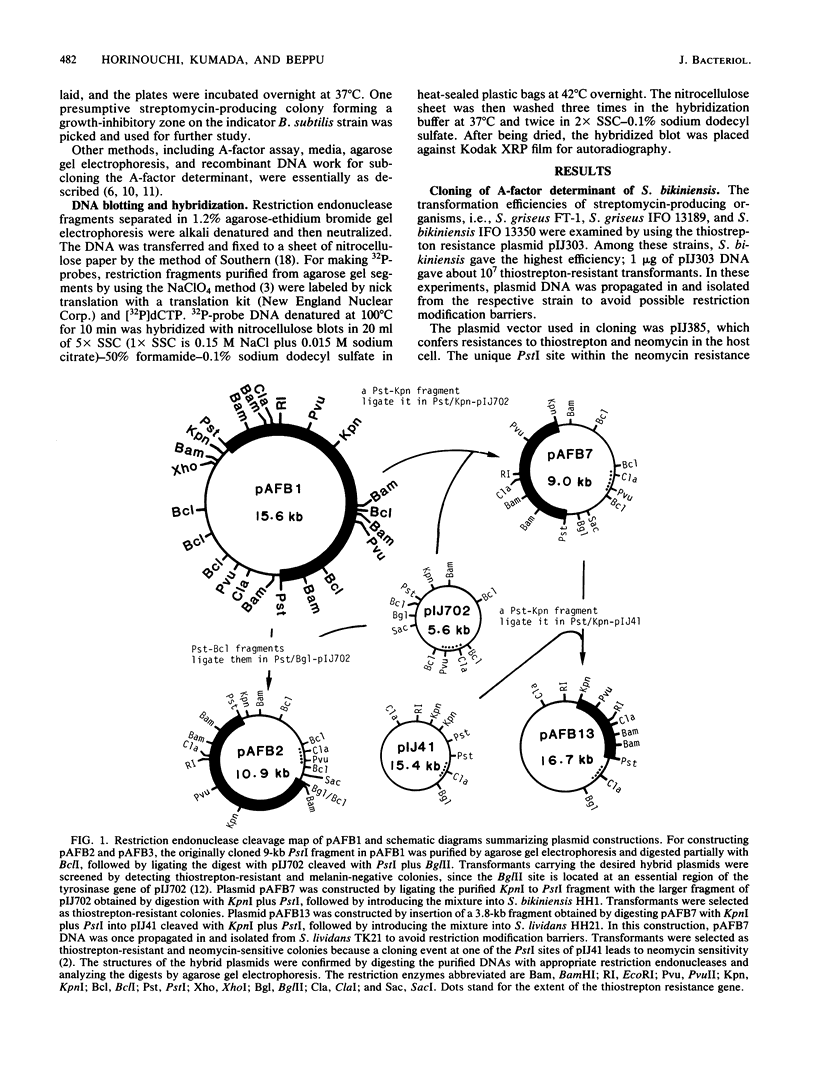
We cloned a DNA fragment directing synthesis of A-factor from the total cellular DNA of streptomycin-producing Streptomyces bikiniensis on the plasmid vector pIJ385 . Introduction of the recombinant plasmid ( pAFB1 ) into A-factor-deficient S. bikiniensis and Streptomyces griseus mutants led to A-factor production in the host cells, as a result of which streptomycin production, streptomycin resistance, and spore formation of these mutants were simultaneously restored. The plasmid pAFB1 also complemented both afsA and afsB mutations of Streptomyces coelicolor A3(2). These results indicated that the cloned DNA fragment contained the genetic determinant of A-factor biosynthesis. The cloned fragment, when carried on a multicopy vector plasmid, induced production of a large amount of A-factor in several Streptomyces hosts. In Southern blot DNA/DNA hybridization analyses with a trimmed 5-kilobase fragment containing the intact A-factor determinant as probe, total cellular DNA from A-factor-deficient mutants gave no positive hybridization. The DNA blot experiment also showed a wide distribution of sequences homologous to the S. bikiniensis A-factor determinant among most, but not all, A-factor-producing actinomycetes with a varying extent of homology and the absence of these sequences from most A-factor nonproducers .
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagawa H., Okanishi M., Umezawa H. A plasmid involved in chloramphenicol production in Streptomyces venezuelae: evidence from genetic mapping. J Gen Microbiol. 1975 Oct;90(2):336–346. doi: 10.1099/00221287-90-2-336. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Hopwood D. A., Kieser T., Thompson C. J. Gene cloning in Streptomyces. Curr Top Microbiol Immunol. 1982;96:69–95. doi: 10.1007/978-3-642-68315-2_5. [DOI] [PubMed] [Google Scholar]
- Chen C. W., Thomas C. A., Jr Recovery of DNA segments from agarose gels. Anal Biochem. 1980 Jan 15;101(2):339–341. doi: 10.1016/0003-2697(80)90197-9. [DOI] [PubMed] [Google Scholar]
- Crameri R., Kieser T., Ono H., Sanchez J., Hütter R. Chromosomal instability in Streptomyces glaucescens: mapping of streptomycin-sensitive mutants. J Gen Microbiol. 1983 Feb;129(2):519–527. doi: 10.1099/00221287-129-2-519. [DOI] [PubMed] [Google Scholar]
- Efremenkova O. V., Anisova L. N., Khokhlov A. S. Vydelenie fazlichnymi aktinomitsetami veshchestv, vyzyvaiushchikh sporuliatsiiu u asporogennogo mutanta Streptomyces griseus. Mikrobiologiia. 1979 Nov-Dec;48(6):999–1003. [PubMed] [Google Scholar]
- Hara O., Beppu T. Induction of streptomycin-inactivating enzyme by A-factor in Streptomyces griseus. J Antibiot (Tokyo) 1982 Sep;35(9):1208–1215. doi: 10.7164/antibiotics.35.1208. [DOI] [PubMed] [Google Scholar]
- Hara O., Beppu T. Mutants blocked in streptomycin production in Streptomyces griseus - the role of A-factor. J Antibiot (Tokyo) 1982 Mar;35(3):349–358. doi: 10.7164/antibiotics.35.349. [DOI] [PubMed] [Google Scholar]
- Hara O., Horinouchi S., Uozumi T., Beppu T. Genetic analysis of A-factor synthesis in Streptomyces coelicolor A3(2) and Streptomyces griseus. J Gen Microbiol. 1983 Sep;129(9):2939–2944. doi: 10.1099/00221287-129-9-2939. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Byeon W. H., Weisblum B. A complex attenuator regulates inducible resistance to macrolides, lincosamides, and streptogramin type B antibiotics in Streptococcus sanguis. J Bacteriol. 1983 Jun;154(3):1252–1262. doi: 10.1128/jb.154.3.1252-1262.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Hara O., Beppu T. Cloning of a pleiotropic gene that positively controls biosynthesis of A-factor, actinorhodin, and prodigiosin in Streptomyces coelicolor A3(2) and Streptomyces lividans. J Bacteriol. 1983 Sep;155(3):1238–1248. doi: 10.1128/jb.155.3.1238-1248.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Khokhlov A. S., Anisova L. N., Tovarova I. I., Kleiner E. M., Kovalenko I. V., Krasilnikova O. I., Kornitskaya E. Y., Pliner S. A. Effect of A-factor on the growth of asporogenous mutants of Streptomyces griseus, not producing this factor. Z Allg Mikrobiol. 1973;13(8):647–655. doi: 10.1002/jobm.3630130803. [DOI] [PubMed] [Google Scholar]
- Kieser T., Hopwood D. A., Wright H. M., Thompson C. J. pIJ101, a multi-copy broad host-range Streptomyces plasmid: functional analysis and development of DNA cloning vectors. Mol Gen Genet. 1982;185(2):223–228. doi: 10.1007/BF00330791. [DOI] [PubMed] [Google Scholar]
- Kirby R., Wright L. F., Hopwood D. A. Plasmid-determined antibiotic synthesis and resistance in Streptomyces coelicolor. Nature. 1975 Mar 20;254(5497):265–267. doi: 10.1038/254265a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Kieser T., Ward J. M., Hopwood D. A. Physical analysis of antibiotic-resistance genes from Streptomyces and their use in vector construction. Gene. 1982 Nov;20(1):51–62. doi: 10.1016/0378-1119(82)90086-5. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Ward J. M., Hopwood D. A. DNA cloning in Streptomyces: resistance genes from antibiotic-producing species. Nature. 1980 Jul 31;286(5772):525–527. doi: 10.1038/286525a0. [DOI] [PubMed] [Google Scholar]