Abstract
The Annelida, which includes the polychaetes and the clitellates, has long held the taxonomic rank of phylum. The unsegmented, mud-dwelling echiuran spoon worms and the gutless, deep-sea pogonophoran tube worms (including vestimentiferans) share several embryological and morphological features with annelids, but each group also has been considered as a separate metazoan phylum based on the unique characters each group displays. Phylogenetic analyses of DNA sequences from the nuclear gene elongation factor-1α place echiurans and pogonophorans within the Annelida. This result, indicating the derived loss of segmentation in echiurans, has profound implications for our understanding of the evolution of metazoan body plans and challenges the traditional view of the phylum-level diversity and evolutionary relationships of protostome worms.
For well over a century, the classification of polychaetes and clitellates (oligochaetes and leeches) as the phylum Annelida has remained stable and widely accepted (1). Paradoxically, no diagnostic characters uniting this group have been identified (1–4), and debate over the affinities of several groups with the annelids has brought the monophyly of the phylum Annelida into question (4, 5). The outcome of this debate has far-reaching implications for the evolution of segmentation and the diversification of protostome coelomates. In assessing whether the phylum Annelida is a natural group that includes all descendants from a common ancestor, resolution of the phylogenetic position of the Echiura and the Pogonophora is critical. If echiurans are in fact modified annelids and do not represent the unique body plan that their phylum status implies, then segmentation is an evolutionarily labile body plan character that has been lost rather than never gained by echiurans. Furthermore, if the Annelida includes the echiurans and the pogonophorans, then the phylum-level diversity of protostome worms and the evolutionary relationships among them are contrary to the diversity and relationships reflected in traditional metazoan classification.
Echiurans are unsegmented, coelomate marine worms that burrow in soft sediments and feed on detritus using a scoop-like proboscis. The lack of segmentation in echiurans has been considered a primitive absence rather than a derived loss (1–7). The absence of this character, along with the presence of unique anal vesicles, proboscis, and muscle layers justified the elevation of echiurans to a separate phylum (6); this taxonomic status of the group is now entrenched in metazoan classification schemes (1–7). Phylogenetic analyses using morphological and embryological data have supported the placement of echiurans as basal to segmented protostomes; however, monophyly of the annelid classes Polychaeta and Clitellata is assumed a priori in these studies, thus ruling out the possible placement of echiurans within the Annelida (3, 4). In the only previous molecular analysis to examine the phylogenetic position of echiurans, which was based on 18S rRNA, no conclusion could be reached regarding the relationship of the single representative species to segmented protostomes (8).
In pogonophorans, both larval and adult features also suggest annelid affinities, and the phylogenetic position of this group of worms has been in contention ever since they were discovered. Pogonophorans are tentaculate, segmented, tube-dwelling worms that are found at ocean depths of 200–10,000 m; adults are gutless, apparently deriving their nutrition from chemoautotrophic endosymbiotic bacteria (9, 10). A new phylum was erected for these bizarre worms in 1944, and, although opinion is divided on the subject, this taxonomic rank remains (1–3, 11, 12). In studying the unsegmented anterior fragments of these animals, early researchers believed that pogonophorans were deuterostomes; however, the protostome condition of these worms became clear with the discovery of the segmented posterior end of the worms and after studies of their embryology (13–17). The vestimentiferan worms of deep sea hydrothermal vents, initially assigned to a separate phylum (18), are now included as a class within the Pogonophora (3–5, 12). To date, phylogenetic analyses of the position and taxonomic status of pogonophorans based on morphological and molecular data have been inconclusive or contradictory (2–4, 8, 19).
The systematics of these groups has remained in doubt or disarray for many reasons: The fossil record provides limited insight into the pattern of radiation for the soft-bodied worms of interest here, possible traces of which have been reported from the Ediacaran and the early-mid Cambrian (20); phylogenetically informative morphological characters at the phylum level are rare and their interpretation controversial (3–5); and molecular analyses have so far focused on 18S rRNA, which has provided inadequate resolution of early protostome divergences (8, 21, 22).
In analyzing the phylogenetic relationships among annelids, echiurans, and pogonophorans, nucleotide sequence data from elongation factor 1α (EF-1α) was chosen for this study. EF-1α is a highly conserved nuclear coding gene that recently has been shown to hold great promise for resolution of deep level divergences among metazoans (23, 24).
MATERIALS AND METHODS
A 346-bp coding region of EF-1α from 10 species was sequenced; these data and 10 additional sequences from the GenBank database were aligned and analyzed using maximum parsimony. The 346-bp coding region of EF-1α was amplified using degenerate primers (19) and PCR (35 cycles: 30 s at 95°C, 1 min at 50–55°C, 1 min 30 s at 72°C; 1.5–4 mM MgCl2); amplifications were from whole genomic preparations (25) or from cDNA preparations, for which RNA was isolated and purified using Ultraspec (Biotecx Laboratories, Houston), and first strand cDNA was synthesized using a poly-T primer with Superscript II (GIBCO/BRL). The PCR product was directly cloned using the TA Cloning Kit (Invitrogen) and was manually sequenced using Sequenase, Ver. 2.0 DNA Polymerase (United States Biochemical). The following taxa were sequenced by the author: Axiothella rubrocincta (maldanid polychaete), Nereis virens (nereidid polychaete), Harmothoe imbricata (polynoid polychaete), Lumbricus terrestris (oligochaete clitellate), Placobdella parasitica (hirudinean clitellate), Ridgeia piscesae (obturate pogonophoran), Listriolobus pelodes (echiuran), Urechis caupo (echiuran), Mytilus sp. (bivalve mollusc), and Cerebratulus sp. (nemertean). The remaining sequences are from GenBank: Lamellibrachia sp. (obturate pogonophoran, D14972), Sternaspis scutata (sternaspid polychaete, D14973), Pheretima sp. (oligochaete clitellate, D14974), Alvinoconcha hessleri (gastropod mollusc, D14975), Apis mellifera (hymenopteran insect, X52884), Bombyx mori (lepidopteran insect, D13338), Rhynchosciara americana (dipteran insect, X66131), Artemia sp. (branchiopod crustacean, X03349 J01165 X00546), Onchocerca volvulus (spirurian nematode, M64333), and Xenopus laevis (amphibian chordate, M25504). Pairwise sequence divergence (for first and second codon positions only) ranges from 0.013 between the clitellates Lumbricus terrestris and Pheretima sp. to 0.24 between the pogonophoran Lamellibrachia sp. and the insect Rhynchosciara americana.
The sequence data for 20 taxa were aligned using LaserGene (DNAstar, Madison, WI) and analyzed using a paup (26) heuristic search (100 replicates, random addition search option; first and second codon positions only; 86 parsimony informative sites).
RESULTS AND DISCUSSION
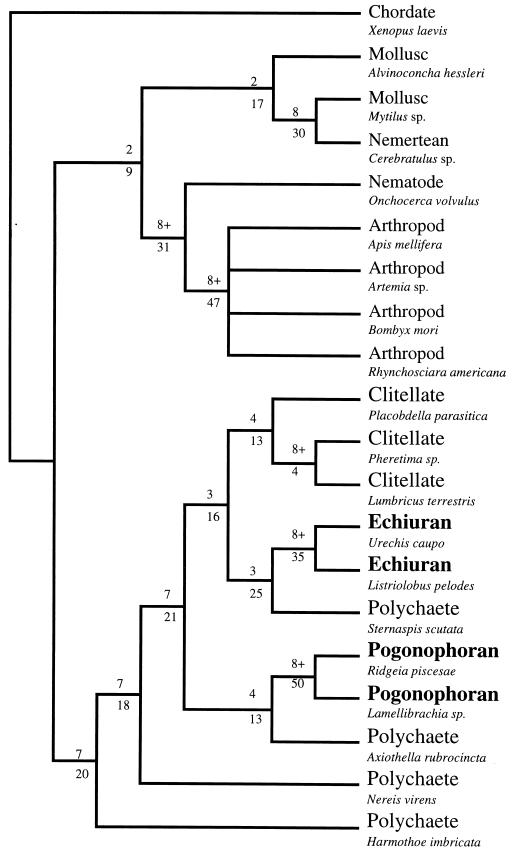
The analysis yielded two equally parsimonious trees (length = 993; consistency index = 0.525; retention index = 0.522) in which echiurans and pogonophorans nest within a clade containing polychaete and clitellate annelids (Fig. 1). The echiurans, the pogonophorans, and the clitellates each form a monophyletic clade within a paraphyletic grade of polychaetes. This renders the traditionally defined Annelida paraphyletic and the phylum status of echiurans and pogonophorans obsolete. Decay indices, ranging from 3 to >8 (Fig. 1), support these results, and topology-dependent cladistic permutation tail probability tests (29) corroborate monophyly of all groups in the worm clade (P < 0.01), including the echiurans plus sternaspid polychaete clade and the pogonophorans plus maldanid polychaete clade. Moreover, if the maximum parsimony analysis is constrained to the traditional classification of the Annelida, i.e., polychaetes plus clitellates, the resultant trees are 32 steps longer than the most parsimonious trees; a Wilcoxon signed rank test (30) shows this result to be highly significant (P < 0.01).
Figure 1.
Results of the phylogenetic analysis showing that echiurans and pogonophorans are derived groups within the annelid clade. The tree shown is the strict consensus of two equally parsimonious trees obtained with a paup (26) heuristic search (100 replicates, random addition search option) on the EF-1α nucleotide sequence data for 20 taxa (first and second codon positions only; 86 parsimony informative sites; length = 993; consistency index = 0.525; retention index = 0.522). The skewness test statistic for the distribution of tree lengths (27), g1 = −0.53, is significant (P < 0.01). Decay indices and branch lengths are shown above and below each node, respectively. The analysis shown incorporated a 4:1 transition/transversion ratio; ratios ranging from 2:1 to 10:1 yielded the same results. The deuterostome, Xenopus laevis, was designated as the outgroup for this analysis. If molluscs and arthropods are used as outgroups, the same worm clade topology results from maximum parsimony analysis, and neighbor joining analysis using the Kimura two-parameter distance model (28) with a γ correction for among-site rate variation also places echiurans and pogonophorans within a monophyletic worm clade.
In this analysis, the clitellates form a monophyletic group that shares an ancestor with some polychaetes, thus supporting previously proposed theories of clitellate evolution; however, the position of the epifaunal polychaetes Nereis and Harmothoe as basal taxa within the worm clade contradicts the view that the ancestral annelid was a burrowing form (31–34). On the contrary, this result lends support to the proposed ancestral condition of polychaetes as morphologically similar to some extant epifaunal polychaete groups, including aphroditiceans (35). Consideration of the relationships of other metazoan groups awaits a broader taxonomic sampling of EF-1α sequences.
Segmentation has been used as the basis for uniting annelids and arthropods as sister taxa; a rival hypothesis supports a sister relationship between molluscs and annelids, with the exclusion of arthropods, on the basis of the trochophore larva (1, 3, 4, 12, 36). In either scheme, echiurans have been considered as primitively unsegmented. However, the results presented here indicate that the unsegmented echiuran condition is derived from a segmented annelid ancestor. Several morphological and embryological lines of evidence provide additional support for the placement of echiurans within the Annelida. For example, echiuran cleavage patterns, chaetal formation, and sperm ultrastructure closely resemble those of polychaete annelids (6, 37, 38).
Early reports of annelid-like teloblastic development in echiurans, the arrangement of ganglia along the larval nerve cord, rings of larval ectodermal mucous glands, and repeated pairs of nephridia in adults are all characters suggestive of segmentation in echiurans. But they have been dismissed as superficial, transitory features (6, 36). In light of the EF-1α sequence data, further investigation of these features is warranted; the possibility that echiurans represent neotenous annelids, in which development of segmentation is suppressed during growth, must be seriously considered (12). Specifically, comparative studies of the expression patterns of homeobox segmentation genes through the larval development of echiurans and segmented annelids may reveal remnants of an ancestral segmented condition in echiurans.
Throughout their colorful taxonomic history, pogonophorans have been variously allied with annelids and with polychaete annelids in particular (19, 39–41). The results of this EF-1α sequence analysis confirm such an alliance, which is further supported by the similarities between the recently described early embryology of pogonophorans and that of polychaete annelids (14–17). The hooked chaetae of pogonophorans also correspond in detail with those of some tube-dwelling polychaetes, including the sister-taxon of pogonophorans in this analysis (Axiothella rubrocincta), the Maldanidae (42). Moreover, the structural properties and amino acid sequences of pogonophoran and annelid extracellular hemoglobin are very similar (43, 44). Pogonophorans, then, represent a group of annelids in which the post-oral segment is greatly elongated and the gut has been lost in the adult.
In a step toward a classification that reflects phylogeny of protostome worms, the Annelida can be redefined to include the echiurans, pogonophorans, clitellates, and polychaetes. The Annelida can then be identified among the protostomes by the presence of paired chitinous chaetae. The “annelid cross” cleavage pattern of blastomeres 1a112–1d112 occurs in echiurans, clitellates, and polychaetes (6, 45, 46) and may constitute another synapomorphy for the Annelida; the pattern of cleavage in pogonophorans has not yet been fully described. A traditional trademark of annelids, segmentation, has been lost in echiurans. Retention of the taxon Polychaeta is not logically consistent with the phylogeny presented here, and it is proposed that the term “polychaete” be used only as an informal reference for a grade of marine annelids. As clades within the Annelida, the echiurans revert to their original name, the Echiuridae, and the pogonophorans assume the name erected in the first species description for the group, the Siboglinidae (47, 48).
Fundamentally different animal body plans, or phyla, constitute groups that are assumed to maintain their phylogenetic integrity as far back as they can be traced (49). Understanding the evolution of metazoan development, morphology, and diversity hinges on this assumption, so it is crucial to examine the monophyly of phyla and the relationships among them. The phylogeny reconstructed from EF-1α sequences demonstrates that the phylum Annelida as traditionally defined is not monophyletic; the results support placement of the echiurans and pogonophorans within the Annelida. These results compel recognition that some characters taken to define body plans, such as segmentation, are more evolutionarily labile than has been generally considered and that the traditional classification of metazoan phyla does not reflect the phylogenetic relationships of protostome worms.
Acknowledgments
I thank L. Katz, S. Bogdanowicz, and R. Harrison for their help and advice throughout this study, and V. Tunnicliffe and C. D. Harvell for specimens. I also thank C. Cavanaugh, M. Hare, L. Katz, O. Oerlemans, J. Pearse, V. Pearse, W. Piel, P. Reynolds, and three anonymous reviewers for their comments on the manuscript. This work was supported by a National Science Foundation/Sloan Foundation Postdoctoral Fellowship in Molecular Evolution through the Section of Ecology and Systematics, Cornell University, Ithaca, NY.
ABBREVIATION
- EF-1α
elongation factor 1α
Footnotes
Data deposition: The sequences presented in this paper have been deposited in the GenBank database (accession nos. AF005498–AF005507).
References
- 1.Brusca R C, Brusca G J. Invertebrates. Sunderland, MA: Sinauer; 1990. [Google Scholar]
- 2.Ax P. The Phylogenetic System: The Systematization of Organisms on the Basis of their Phylogenesis. New York: Wiley; 1987. [Google Scholar]
- 3.Eernisse D J, Albert J S, Anderson F E. Syst Biol. 1992;41:305–330. [Google Scholar]
- 4.Rouse G W, Fauchald K. Zool Scripta. 1995;24:269–301. [Google Scholar]
- 5.Nielsen C. Animal Evolution: Interrelationships of the Living Phyla. Oxford: Oxford Univ. Press; 1995. [Google Scholar]
- 6.Newby W W. Mem Am Phil Soc. 1940;16:1–219. [Google Scholar]
- 7.Stephen A C, Edmonds S J. The Phyla Sipuncula and Echiura. London: British Museum Natural History; 1972. [Google Scholar]
- 8.Winnepenninckx B, Backeljau T, De Wachter R. Mol Biol Evol. 1995;12:641–649. doi: 10.1093/oxfordjournals.molbev.a040243. [DOI] [PubMed] [Google Scholar]
- 9.Southward E C. In: Microscopic Anatomy of Invertebrates. Harrison F W, Rice M E, editors. Vol. 12. New York: Wiley–Liss; 1993. pp. 327–369. [Google Scholar]
- 10.Cavanaugh C M. Am Zool. 1994;34:79–89. [Google Scholar]
- 11.Beklemishev V N. Principles of Comparative Anatomy of Invertebrates. Moscow: Akademia Nauk; 1944. (in Russian). [Google Scholar]
- 12.Pearse V, Pearse J, Buchsbaum M, Buchsbaum R. Living Invertebrates. Pacific Grove, CA: Boxwood; 1987. [Google Scholar]
- 13.Ivanov A V. Syst Zool. 1954;3:69–79. , (1955) 4, 171–177. [Google Scholar]
- 14.Southward E C. J Mar Biol Assoc UK. 1988;68:465–487. [Google Scholar]
- 15.Gardiner S L, Jones M L. Am Zool. 1994;34:513–522. [Google Scholar]
- 16.Callsen-Cencic P, Flügel H J. Sarsia. 1995;80:73–89. [Google Scholar]
- 17.Young C M, Vásquez E, Metaxas A, Tyler P A. Nature (London) 1996;381:514–516. [Google Scholar]
- 18.Jones M L. Bull Biol Soc Wash. 1985;6:117–158. [Google Scholar]
- 19.Kojima S, Hashimoto T, Hasegawa M, Murata S, Ohta S, Seki H, Okada N. J Mol Evol. 1993;37:66–70. doi: 10.1007/BF00170463. [DOI] [PubMed] [Google Scholar]
- 20.Benton M J. The Fossil Record. London: Chapman & Hall; 1993. [Google Scholar]
- 21.Philippe, H., Chenuil, A. & Adoutte, A. (1994) Development 1994, Suppl., 15–25.
- 22.Winnepenninckx B, Backeljau T, De Wachter R. Mol Biol Evol. 1996;13:1306–1317. doi: 10.1093/oxfordjournals.molbev.a025577. [DOI] [PubMed] [Google Scholar]
- 23.Friedlander T P, Regier J C, Mitter C. Syst Biol. 1994;43:511–525. [Google Scholar]
- 24.Kobayashi M, Wada H, Satoh N. Mol Phylo Evol. 1996;5:414–422. doi: 10.1006/mpev.1996.0036. [DOI] [PubMed] [Google Scholar]
- 25.Winnepenninckx B, Backeljau T, De Wachter R. Trends Genet. 1993;9:407.20. doi: 10.1016/0168-9525(93)90102-n. [DOI] [PubMed] [Google Scholar]
- 26.Swofford D. paup: Phylogenetic Analysis Using Parsimony, Ver. 3.1.1. Champaign, IL: Illinois Natural History Survey; 1993. [Google Scholar]
- 27.Hillis D M, Huelsenbeck J P. J Hered. 1992;83:189–195. doi: 10.1093/oxfordjournals.jhered.a111190. [DOI] [PubMed] [Google Scholar]
- 28.Kimura M. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 29.Faith D P. Syst Zool. 1991;40:366–375. [Google Scholar]
- 30.Templeton A R. Evolution. 1983;37:221–244. doi: 10.1111/j.1558-5646.1983.tb05533.x. [DOI] [PubMed] [Google Scholar]
- 31.Clark R B. Dynamics in Metazoan Evolution. Oxford: Clarendon Press; 1964. [Google Scholar]
- 32.Clark R B. In: Chemical Zoology. Florkin M, Scheer B T, editors. Vol. 4. New York: Academic; 1969. pp. 1–68. [Google Scholar]
- 33.Brinkhurst R O, Jamieson B G M. Aquatic Oligochaeta of the World. Edinburgh: Oliver and Boyd; 1971. [Google Scholar]
- 34.Fauchald K. Syst Zool. 1975;23:493–506. [Google Scholar]
- 35.Conway Morris S, Peel J S. Phil Trans R Soc Lond B. 1995;347:305–358. [Google Scholar]
- 36.Ghiselin M T. Oxford Surv Evol Biol. 1988;5:66–95. [Google Scholar]
- 37.Franzén Å, Ferraguti M. Acta Zool. 1992;73:25–31. [Google Scholar]
- 38.Pilger J F. In: Microscopic Anatomy of Invertebrates. Harrison F W, Rice M E, editors. Vol. 12. New York: Wiley–Liss; 1993. pp. 185–236. [Google Scholar]
- 39.Uschakov P. Zool Anz. 1933;104:205–208. [Google Scholar]
- 40.Webb M. Bull Mar Sci. 1969;19:18–47. [Google Scholar]
- 41.van der Land F, Nørrevang A. Biol Skr Dan Vid Selsk. 1977;21:1–102. [Google Scholar]
- 42.Bartolomaeus T. Zoomorphology. 1995;115:161–177. [Google Scholar]
- 43.Terwilliger R C, Terwilliger N B, Bonaventura C, Bonaventura J, Schabtach E. Biochim Biophys Acta. 1985;829:27–33. [Google Scholar]
- 44.Suzuki T, Takagi T, Okuda K, Furukohri T, Ohta S. Zool Sci. 1989;6:915–926. [Google Scholar]
- 45.Salvini-Plawen L von. Microfauna Marina. 1988;4:383–396. [Google Scholar]
- 46.Needham A E. In: Reproductive Biology of Invertebrates. Adiyodi K G, Adiyodi R G, editors. Vol. 5. New York: Wiley; 1990. pp. 1–36. [Google Scholar]
- 47.Hatschek B. Arbeit Zool Inst Wien. 1880;3:45–78. [Google Scholar]
- 48.Caullery M. Bull Soc Zool Fr. 1914;39:350–353. [Google Scholar]
- 49.Conway Morris, S. (1994) Development 1994, Suppl., 1–13.