Abstract
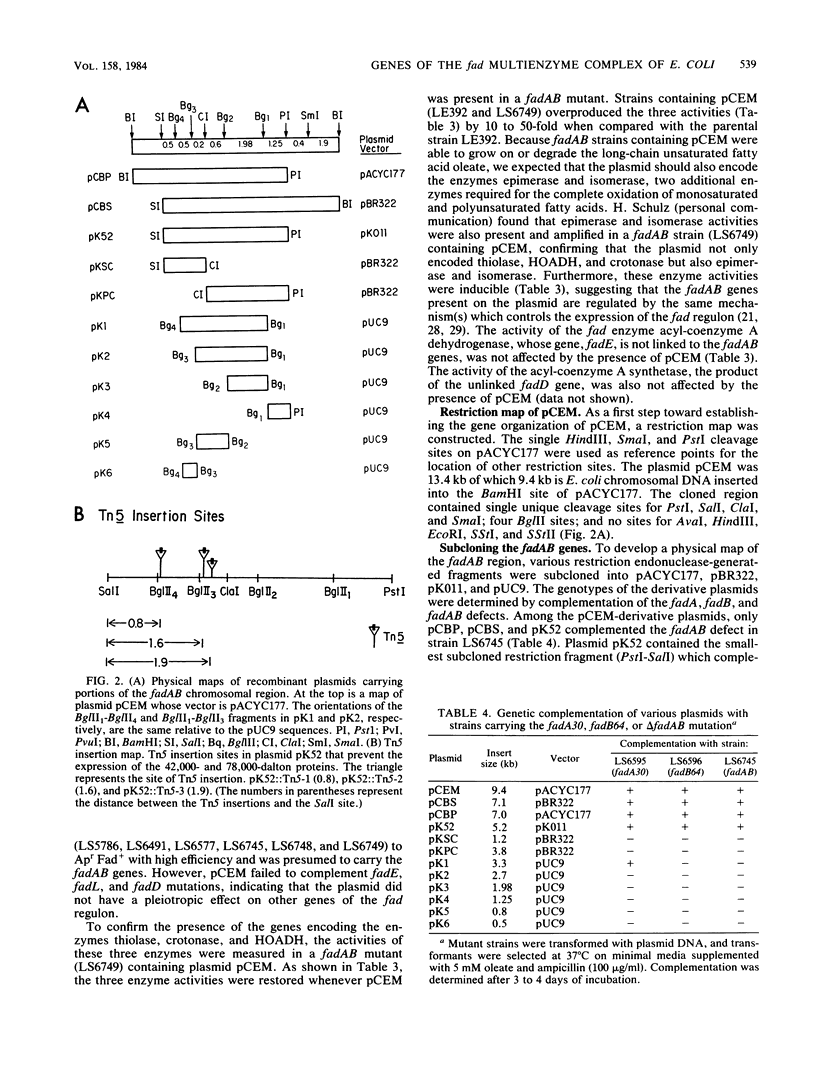
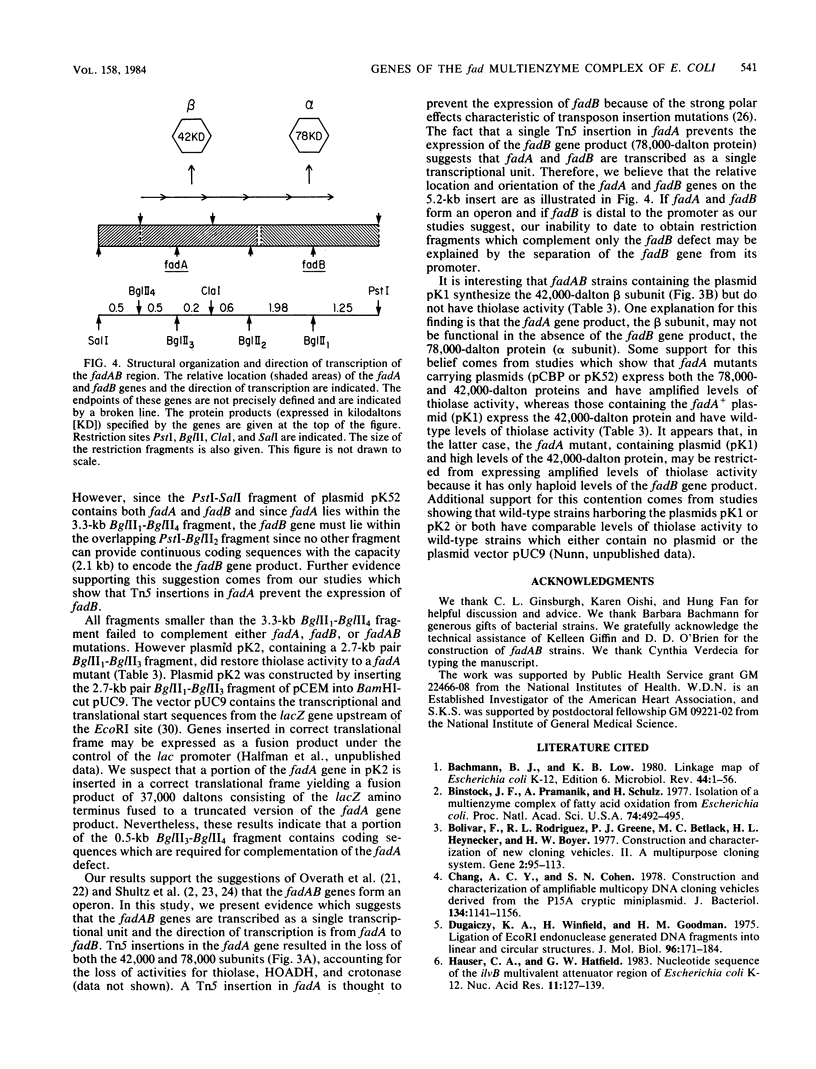
Two protein subunits (42,000 and 78,000 daltons) encoded by the fadAB genes form a multifunctional enzyme complex containing thiolase, 3-hydroxyacyl-coenzyme A dehydrogenase, crotonase , epimerase, and isomerase activities (S. Pawar and H. Schulz, J. Biol. Chem. 256:3894-3899, 1981). In an attempt to characterize the structural organization and regulatory properties of these genes, a 5.2-kilobase pair fragment containing the fadAB genes has been isolated. Plasmids containing this fragment (i) complement mutations in the fadAB genes; (ii) overproduce by 10- to 50-fold thiolase, 3-hydroxyacyl-coenzyme A dehydrogenase and crotonase ; and (iii) specify a 42,000- and a 78,000-dalton protein. The fadA gene, which encodes the 42,000-dalton protein, has been localized within the original clone to a 3.3-kilobase pair fragment. Thiolase activity, which is encoded by the 42,000-dalton protein, was not observed in the absence of the 78,000-dalton protein, suggesting that an intact complex is required for function. Transposon Tn5 insertional mutagenesis of the cloned fadAB genes has demonstrated that both fadA and fadB are transcribed as a single transcriptional unit with the direction of transcription from fadA to fadB . The molecular cloning and characterization of the fadAB region confirm the original genetic contention that the genes encoding the proteins for the multifunctional complex form an operon.
Full text
PDF
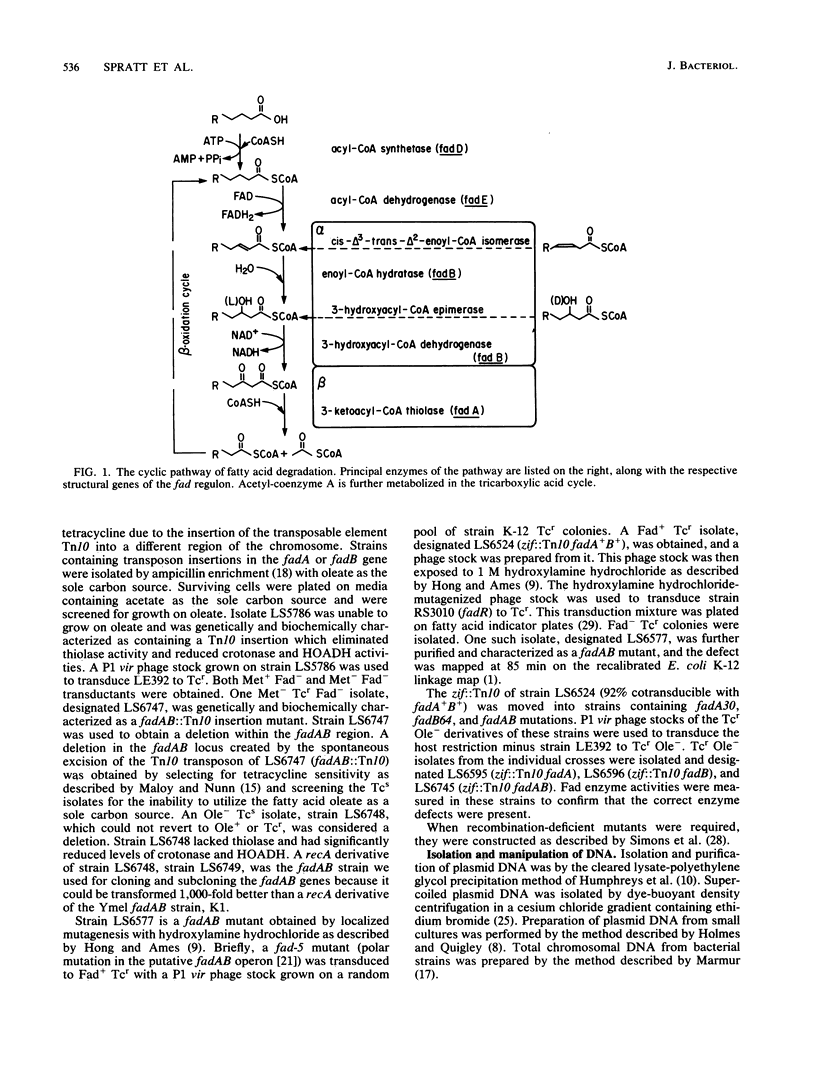
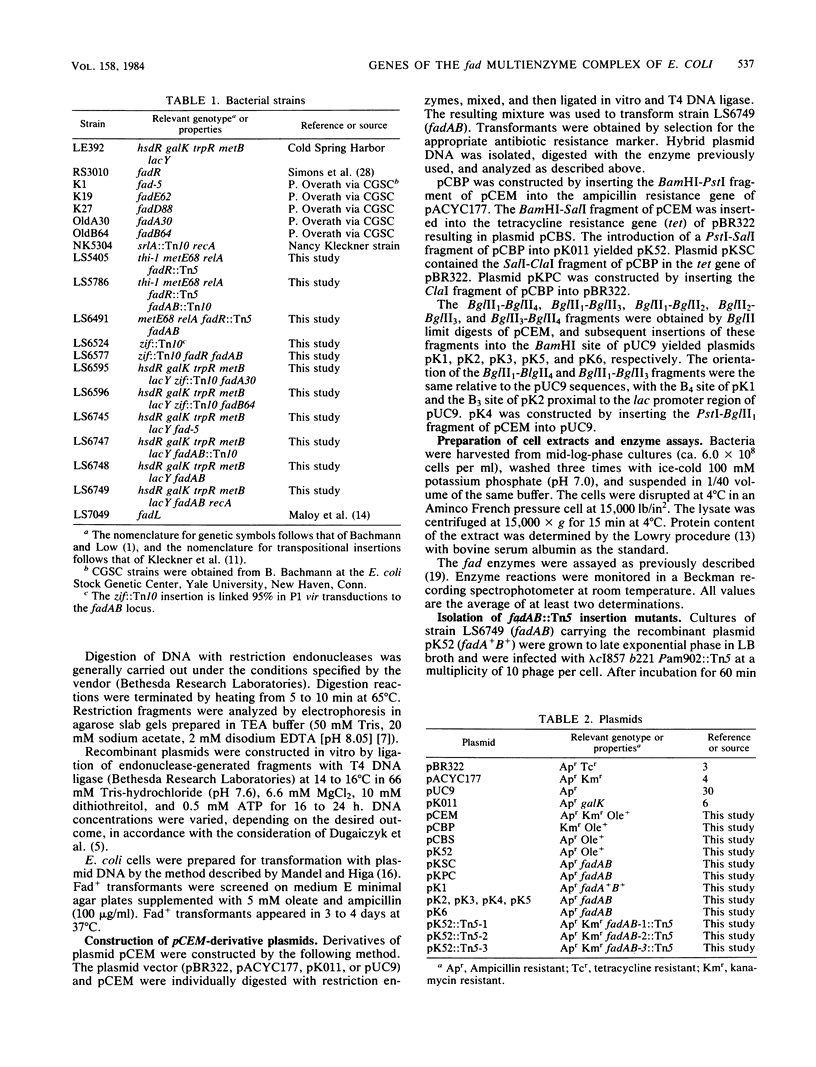
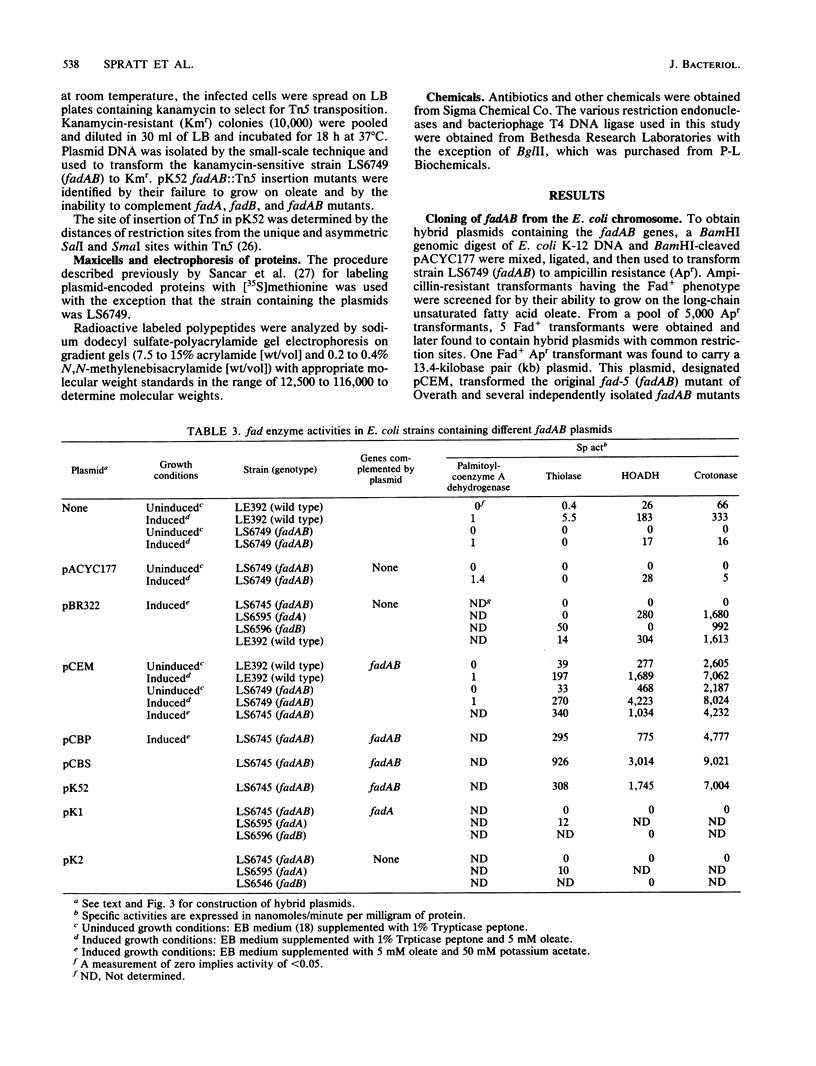


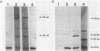
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binstock J. F., Pramanik A., Schulz H. Isolation of a multi-enzyme complex of fatty acid oxidation from Escherichia coli. Proc Natl Acad Sci U S A. 1977 Feb;74(2):492–495. doi: 10.1073/pnas.74.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Hauser C. A., Hatfield G. W. Nucleotide sequence of the ilvB multivalent attenuator region of Escherichia coli K12. Nucleic Acids Res. 1983 Jan 11;11(1):127–139. doi: 10.1093/nar/11.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Ames B. N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Klein K., Steinberg R., Fiethen B., Overath P. Fatty acid degradation in Escherichia coli. An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur J Biochem. 1971 Apr;19(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01334.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maloy S. R., Ginsburgh C. L., Simons R. W., Nunn W. D. Transport of long and medium chain fatty acids by Escherichia coli K12. J Biol Chem. 1981 Apr 25;256(8):3735–3742. [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Nunn W. D., Simons R. W., Egan P. A., Maloy S. R. Kinetics of the utilization of medium and long chain fatty acids by mutant of Escherichia coli defective in the fadL gene. J Biol Chem. 1979 Sep 25;254(18):9130–9134. [PubMed] [Google Scholar]
- O'Brien W. J., Frerman F. E. Evidence for a complex of three beta-oxidation enzymes in Escherichia coli: induction and localization. J Bacteriol. 1977 Nov;132(2):532–540. doi: 10.1128/jb.132.2.532-540.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Pauli G., Schairer H. U. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur J Biochem. 1969 Feb;7(4):559–574. [PubMed] [Google Scholar]
- Overath P., Raufuss E. M. The induction of the enzymes of fatty acid degradation in Escherichia coli. Biochem Biophys Res Commun. 1967 Oct 11;29(1):28–33. doi: 10.1016/0006-291x(67)90535-9. [DOI] [PubMed] [Google Scholar]
- Pawar S., Schulz H. The structure of the multienzyme complex of fatty acid oxidation from Escherichia coli. J Biol Chem. 1981 Apr 25;256(8):3894–3899. [PubMed] [Google Scholar]
- Pramanik A., Pawar S., Antonian E., Schulz H. Five different enzymatic activities are associated with the multienzyme complex of fatty acid oxidation from Escherichia coli. J Bacteriol. 1979 Jan;137(1):469–473. doi: 10.1128/jb.137.1.469-473.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein S. J., Jorgensen R. A., Postle K., Reznikoff W. S. The inverted repeats of Tn5 are functionally different. Cell. 1980 Mar;19(3):795–805. doi: 10.1016/s0092-8674(80)80055-9. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Egan P. A., Chute H. T., Nunn W. D. Regulation of fatty acid degradation in Escherichia coli: isolation and characterization of strains bearing insertion and temperature-sensitive mutations in gene fadR. J Bacteriol. 1980 May;142(2):621–632. doi: 10.1128/jb.142.2.621-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Hughes K. T., Nunn W. D. Regulation of fatty acid degradation in Escherichia coli: dominance studies with strains merodiploid in gene fadR. J Bacteriol. 1980 Aug;143(2):726–730. doi: 10.1128/jb.143.2.726-730.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weeks G., Shapiro M., Burns R. O., Wakil S. J. Control of fatty acid metabolism. I. Induction of the enzymes of fatty acid oxidation in Escherichia coli. J Bacteriol. 1969 Feb;97(2):827–836. doi: 10.1128/jb.97.2.827-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]