Abstract
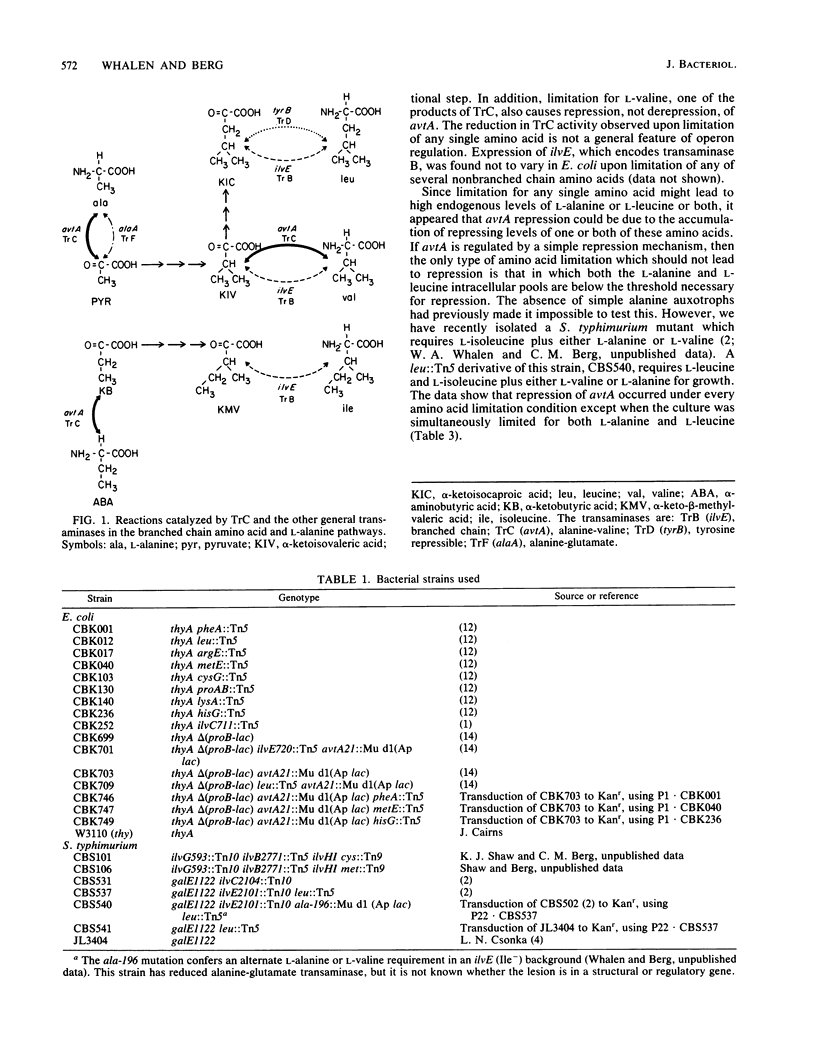
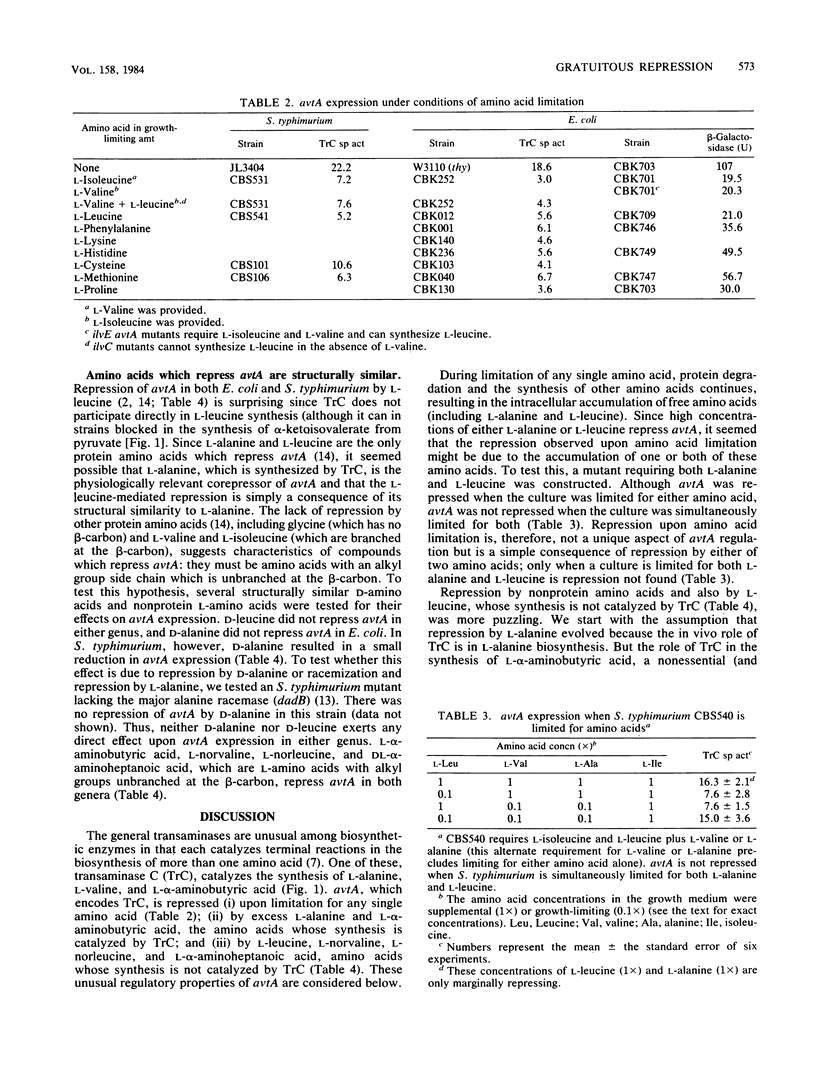
avtA , which encodes transaminase C (alanine-valine transaminase), is repressed by excess-L-alanine or L-leucine, and also by limitation for any of a number of amino acids in Escherichia coli and Salmonella typhimurium. Amino acid limitation causes repression by promoting the accumulation of L-alanine or L-leucine or both. avtA is also repressed by L-alpha-aminobutyric acid and other nonprotein amino acids which are structurally similar to L-alanine. We hypothesize that L-alanine and L-alpha-aminobutyric acid, whose syntheses are catalyzed by transaminase C, are the true corepressors of avtA . Repression by structural analogs of the true corepressors is termed gratuitous repression.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg C. M., Shaw K. J., Vender J., Borucka-Mankiewicz M. Physiological characterization of polar Tn5-induced isoleucine-valine auxotrophs in Escherichia coli K.12: evidence for an internal promoter in the ilvOGEDA operon. Genetics. 1979 Oct;93(2):308–319. [PMC free article] [PubMed] [Google Scholar]
- Berg C. M., Whalen W. A., Archambault L. B. Role of alanine-valine transaminase in Salmonella typhimurium and analysis of an avtA::Tn5 mutant. J Bacteriol. 1983 Sep;155(3):1009–1014. doi: 10.1128/jb.155.3.1009-1014.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N., Howe M. M., Ingraham J. L., Pierson L. S., 3rd, Turnbough C. L., Jr Infection of Salmonella typhimurium with coliphage Mu d1 (Apr lac): construction of pyr::lac gene fusions. J Bacteriol. 1981 Jan;145(1):299–305. doi: 10.1128/jb.145.1.299-305.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkinham J. O., 3rd Identification of a mutation affecting an alanine-alpha-ketoisovalerate transaminase activity in Escherichia coli K-12. Mol Gen Genet. 1979 Oct 2;176(1):147–149. doi: 10.1007/BF00334306. [DOI] [PubMed] [Google Scholar]
- Freundlich M. Valyl-Transfer RNA: Role in Repression of the Isoleucine-Valine Enzymes in Escherichia coli. Science. 1967 Aug 18;157(3790):823–825. doi: 10.1126/science.157.3790.823-a. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Calhoun D. H. Intracellular roles of microbial aminotransferases: overlap enzymes across different biochemical pathways. Crit Rev Microbiol. 1981;8(3):229–266. doi: 10.3109/10408418109085080. [DOI] [PubMed] [Google Scholar]
- LEAVITT R. I., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. XI. Valine inhibition of the growth of Escherichia coli strain K-12. J Bacteriol. 1962 Mar;83:624–630. doi: 10.1128/jb.83.3.624-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGilvray D., Umbarger H. E. Regulation of transaminase C synthesis in Escherichia coli: conditional leucine auxotrophy. J Bacteriol. 1974 Nov;120(2):715–723. doi: 10.1128/jb.120.2.715-723.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D. Interrelationships between amino-acids in the growth of coliform organisms. J Gen Microbiol. 1953 Aug;9(1):37–43. doi: 10.1099/00221287-9-1-37. [DOI] [PubMed] [Google Scholar]
- RUDMAN D., MEISTER A. Transamination in Escherichia coli. J Biol Chem. 1953 Feb;200(2):591–604. [PubMed] [Google Scholar]
- Shaw K. J., Berg C. M. Escherichia coli K-12 auxotrophs induced by insertion of the transposable element Tn5. Genetics. 1979 Jul;92(3):741–747. doi: 10.1093/genetics/92.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman S. A., Walsh C. T., Botstein D. Two alanine racemase genes in Salmonella typhimurium that differ in structure and function. J Bacteriol. 1983 Mar;153(3):1439–1450. doi: 10.1128/jb.153.3.1439-1450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen W. A., Berg C. M. Analysis of an avtA::Mu d1(Ap lac) mutant: metabolic role of transaminase C. J Bacteriol. 1982 May;150(2):739–746. doi: 10.1128/jb.150.2.739-746.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


