Abstract
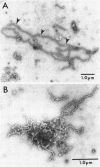
The expression of fumarate reductase in Escherichia coli has been amplified over 30-fold by utilizing a recombinant plasmid, pFR63 , carrying the fumarate reductase operon. More than 50% of the inner-membrane protein could be accounted for by the enzyme, whereas the total amount of protein associated with the membrane fraction doubled. The membrane accommodated this excess fumarate reductase without reducing the levels of other membrane-associated enzymes. At the same time, the amount of membrane lipid increased such that the lipid/protein ratio remained constant, indicating that the total amount of membrane had doubled. Small alterations in fatty acid composition as well as a large increase in cardiolipin were detected in the fumarate reductase-enriched membranes. The excess membrane was localized in novel tubular structures which were observed in thin-section and negatively stained electron-microscopic preparations. The tubules only appeared after the cytoplasmic membrane became highly enriched in fumarate reductase. They branched from the cytoplasmic membrane and were fumarate reductase. They branched from the cytoplasmic membrane and were composed of an aggregate of fumarate reductase and lipid.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chamberlain B. K., Webster R. E. Lipid-protein interactions in Escherichia coli. Membrane-associated f1 bacteriophage coat protein and phospholipid metabolism. J Biol Chem. 1976 Dec 25;251(24):7739–7745. [PubMed] [Google Scholar]
- Cole S. T., Grundström T., Jaurin B., Robinson J. J., Weiner J. H. Location and nucleotide sequence of frdB, the gene coding for the iron-sulphur protein subunit of the fumarate reductase of Escherichia coli. Eur J Biochem. 1982 Aug;126(1):211–216. doi: 10.1111/j.1432-1033.1982.tb06768.x. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Guest J. R. Genetic and physical characterization of lambda transducing phages (lambda frdA) containing the fumarate reductase gene of Escherichia coli K12. Mol Gen Genet. 1980;178(2):409–418. doi: 10.1007/BF00270492. [DOI] [PubMed] [Google Scholar]
- Dickie P., Weiner J. H. Purification and characterization of membrane-bound fumarate reductase from anaerobically grown Escherichia coli. Can J Biochem. 1979 Jun;57(6):813–821. doi: 10.1139/o79-101. [DOI] [PubMed] [Google Scholar]
- Grundström T., Jaurin B. Overlap between ampC and frd operons on the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1111–1115. doi: 10.1073/pnas.79.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lemire B. D., Robinson J. J., Bradley R. D., Scraba D. G., Weiner J. H. Structure of fumarate reductase on the cytoplasmic membrane of Escherichia coli. J Bacteriol. 1983 Jul;155(1):391–397. doi: 10.1128/jb.155.1.391-397.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire B. D., Robinson J. J., Weiner J. H. Identification of membrane anchor polypeptides of Escherichia coli fumarate reductase. J Bacteriol. 1982 Dec;152(3):1126–1131. doi: 10.1128/jb.152.3.1126-1131.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmeier E., Hagen D. S., Dickie P., Weiner J. H. Cloning and expression of fumarate reductase gene of Escherichia coli. Can J Biochem. 1981 Mar;59(3):158–164. doi: 10.1139/o81-023. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Chen C. M., Silver S., Rosen B. P. Cloning and expression of R-factor mediated arsenate resistance in Escherichia coli. Mol Gen Genet. 1983;191(3):421–426. doi: 10.1007/BF00425757. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Ballou C. E. Acyl phosphatidylglycerol. A new phospholipid from Salmonella typhimurium. J Biol Chem. 1971 May 25;246(10):3305–3313. [PubMed] [Google Scholar]
- Saito Y., McElhaney R. N. Membrane lipid biosynthesis in Acholeplasma laidlawii B: incorporation of exogenous fatty acids into membrane glyco- and phospholipids by growing cells. J Bacteriol. 1977 Nov;132(2):485–496. doi: 10.1128/jb.132.2.485-496.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers A., Weiner J. H. The anaerobic sn-glycerol-3-phosphate dehydrogenase of Escherichia coli. Purification and characterization. J Biol Chem. 1981 Oct 10;256(19):9959–9965. [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Proteins of the inner membrane of Escherichia coli: changes in composition associated with anaerobic growth and fumarate reductase amber mutation. J Bacteriol. 1974 Mar;117(3):954–959. doi: 10.1128/jb.117.3.954-959.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J. W., Shapiro B. M. The respiratory chain NADH dehydrogenase of Escherichia coli. Isolation of an NADH:quinone oxidoreductase from membranes and comparison with the membrane-bound NADH:dichlorophenolindophenol oxidoreductase. J Biol Chem. 1981 Mar 25;256(6):3077–3084. [PubMed] [Google Scholar]
- Weiner J. H., Dickie P. Fumarate reductase of Escherichia coli. Elucidation of the covalent-flavin component. J Biol Chem. 1979 Sep 10;254(17):8590–8593. [PubMed] [Google Scholar]
- Yamato I., Futai M., Anraku Y., Nonomura Y. Cytoplasmic membrane vesicles of Escherichia coli. II. Orientation of the vesicles studied by localization of enzymes. J Biochem. 1978 Jan;83(1):117–128. doi: 10.1093/oxfordjournals.jbchem.a131882. [DOI] [PubMed] [Google Scholar]